 |
 |
- Search
| Psychiatry Investig > Volume 17(10); 2020 > Article |
|
Abstract
Objective
Methods
Results
ACKNOWLEDGEMENTS
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Seok Woo Moon. Data curation: Beomjun Kim. Formal analysis: Seok Woo Moon. Funding acquisition: Seok Woo Moon. Investigation: Beomjun Kim, Seok Woo Moon. Methodology: Seok Woo Moon. Project administration: Beomjun Kim, Seok Woo Moon. Resource: Seok Woo Moon. Software: Beomjun Kim. Supervision: Seok Woo Moon. Validation: Beomjun Kim, Seok Woo Moon. Visualization: Beomjun Kim. Writing—original draft: Beomjun Kim. Writing—review&editing: Beomjun Kim, Seok Woo Moon.
Figure 1.
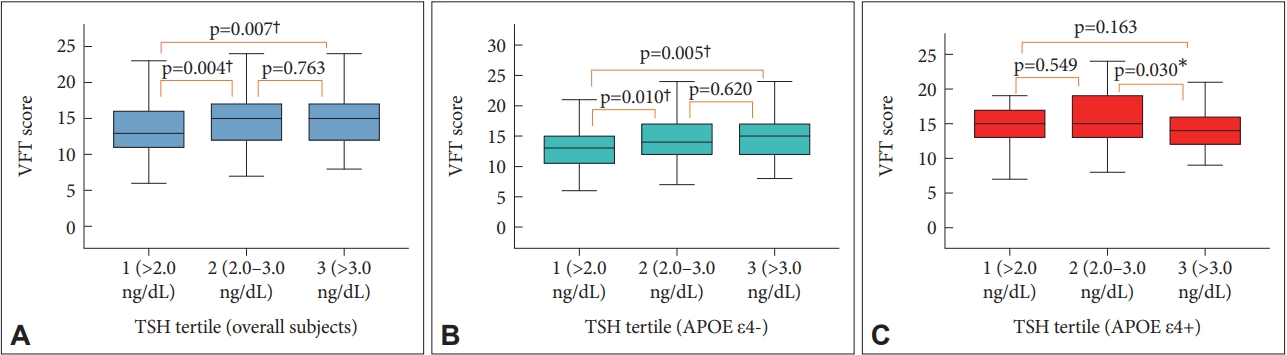
Table 1.
| Total | TSH 1 (<2.0 μIU/mL) | TSH 2 (2.0-3.0 μIU/mL) | TSH 3 (>3.0 μIU/mL) | p-value | |
|---|---|---|---|---|---|
| Participants, N (%) | 507 (100) | 134 (26.2) | 152 (30.0) | 221 (43.6) | |
| TSH (mIU/L), M (SD)‡ | 2.180 (0.82) | 1.193 (0.386) | 2.284 (0.371) | 4.912 (2.956) | 0.001* |
| Age, years, M (SD) | 74.48 (7.957) | 75.16 (7.693) | 73.61 (7.763) | 74.67 (8.225) | 0.231 |
| Gender, male, N (%) | 275 (39.3) | 69 (51.5) | 92 (60.5) | 110 (49.8) | 0.107 |
| Years of education, M (SD) | 6.81 (3.477) | 6.24 (3.648) | 6.54 (3.018) | 7.34 (4.648) | 0.143 |
| Free T4 (ug/dL) | 1.069 (0.259) | 1.133 (0.256) | 1.059 (0.225) | 1.037 (0.277) | 0.003* |
| APOE ε4, N (%) | 100 (19.7) | 26 (19.4) | 33 (21.7) | 41 (18.6) | 0.749 |
| GDS-K total score, M (SD) | 9.97 (6.906) | 10.62 (7.077) | 9.78 (6.669) | 9.70 (6.965) | 0.440 |
Table 2.
|
APOE ε4 status |
TSH level |
APOE ε4 status*TSH level (interaction term) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type III SS | df | MS | F | p value | Type III SS | df | MS | F | p value | Type III SS | df | MS | F | p value | ||
| Model I | ||||||||||||||||
| VFT | 7.968 | 1 | 7.968 | 0.710 | 0.400 | 112.086 | 2 | 56.043 | 4.991 | 0.007* | ||||||
| BNT | 2.797 | 1 | 2.797 | 0.528 | 0.468 | 6.634 | 2 | 3.317 | 0.626 | 0.535 | ||||||
| WLMT | 44.248 | 1 | 44.248 | 4.000 | 0.046† | 77.273 | 2 | 38.637 | 3.493 | 0.031* | ||||||
| CPT | 0.429 | 1 | 0.429 | 0.262 | 0.609 | 2.809 | 2 | 1.405 | 0.860 | 0.424 | ||||||
| WLRT | 17.046 | 1 | 17.046 | 6.108 | 0.014† | 21.307 | 2 | 10.653 | 3.817 | 0.023† | ||||||
| WLRcT | 3.276 | 1 | 3.276 | 1.509 | 0.220 | 6.303 | 2 | 3.152 | 1.452 | 0.235 | ||||||
| CRT | 4.345 | 1 | 4.345 | 0.694 | 0.405 | 19.369 | 2 | 9.685 | 1.547 | 0.214 | ||||||
| Model II | ||||||||||||||||
| VFT | 20.186 | 1 | 20.186 | 1.817 | 0.178 | 146.277 | 2 | 73.139 | 6.584 | 0.002* | 81.999 | 2 | 41.000 | 3.691 | 0.026* | |
| BNT | 2.239 | 1 | 2.239 | 0.421 | 0.517 | 7.392 | 2 | 3.696 | 0.695 | 0.499 | 0.875 | 2 | 0.438 | 0.082 | 0.921 | |
| WLMT | 50.122 | 1 | 50.122 | 4.547 | 0.033† | 56.867 | 2 | 28.434 | 2.579 | 0.077 | 41.059 | 2 | 20.530 | 1.862 | 0.156 | |
| CPT | 0.294 | 1 | 0.294 | 0.180 | 0.671 | 1.033 | 2 | 0.516 | 0.317 | 0.729 | 4.000 | 2 | 2.000 | 1.226 | 0.294 | |
| WLRT | 19.79 | 1 | 19.790 | 7.101 | 0.008† | 11.338 | 2 | 5.669 | 2.034 | 0.132 | 7.578 | 2 | 3.789 | 1.360 | 0.258 | |
| WLRcT | 4.429 | 1 | 4.429 | 2.041 | 0.154 | 5.324 | 2 | 2.662 | 1.227 | 0.294 | 4.892 | 2 | 2.446 | 1.127 | 0.325 | |
| CRT | 4.782 | 1 | 4.782 | 0.761 | 0.383 | 17.927 | 2 | 8.963 | 1.426 | 0.241 | 0.992 | 2 | 0.496 | 0.079 | 0.924 | |
† general linear model (GLM) analysis Model I: APOE and TSH level, Model II: Model I+APOE*TSH level (interaction term).
Age, gender, education and Korean version of Geriatric Depression Scale (GDS-K) adjusted for multiple analysis of covariance (MANOVA). APOE: apolipoprotein E, TSH: thyroid stimulating hormone, SS: square sum, MS: mean squre, VFT: verbal fluency test, mBNT: modified Boston naming test, WLMT: word list memory test, CPT: construction praxis test, WLRT: word list recall test, WLRcT: word list recognition test, CRT: construction recall test
Table 3.
Age, gender, education and Korean version of Geriatric Depression Scale (GDS-K) adjusted for multiple analysis of covariance (MANOVA). SS: square sum, MS: mean squre, VFT: verbal fluency test, mBNT: modified Boston naming test, WLMT: word list memory test, CPT: construction praxis test, WLRT: word list recall test, WLRcT: word list recognition test, CRT: construction recall test
REFERENCES







