INTRODUCTION
C-reactive protein (CRP) is a conserved protein which has existed for more than 400 million years and distributed amongst vertebrate and invertebrate animals. CRP can strongly react with C polysaccharide of pneumococcus and thus get its name [1]. CRP was initially indentified in serum of those patients who got acute bacterial infection [2]. At acute inflammation phase, CRP is majorly produced in liver and its concentration might raise 2000-folds during the first 24-48 hours of infection. Because serum CRP level is barely detectable under healthy condition and usually higher than 10 mg/L in acute inflammation condition, people have long thought relative low level of serum CRP (lower than 10 mg/L) doesn’t represent any physiological or pathological situations. However, recent studies have found under some chronic inflammation conditions, such as cardiovascular disease, metabolic syndrome and cancer, patients would usually have a serum CRP level higher than 3 mg/L [3,4].
It has been widely acknowledged that chronic inflammation is largely involved in depression [5]. Considering elevated CRP is a primary marker for chronic inflammation, two hypotheses in the field have been raised regarding to chronic inflammation and depression [6]. The first hypothesis is chronic inflammation may induce depression as well as increasing serum CRP level in parallel; on contrast, the second hypothesis is chronic inflammation condition will raise serum CRP level, which may than lead to depression by some unveil mechanisms. Plenty of effects have been made to find out more precise mechanism under CRP and depression, however, the results to date were inconsistent and full of controversy.
Interestingly, study from identical twins has suggested both CRP polymorphisms and depression are about 40% inheritable [7]. Furthermore, a recent study has found a potential pathway that serum CRP, instead of chronic inflammation, may cause depression [8]. Besides that, there are also several CRP genetic polymorphisms have been found could regulate serum CRP levels [9]. Considering all above evidences together, in the present study, we raised our hypothesis that CRP polymorphisms which could up-regulate serum CRP levels may positively relate to depression occurrence. To investigate this hypothesis, we recruited depression patients with positive family history and healthy volunteers as control into our project, and analyzed their serum CRP level as well as CRP SNPs distribution.
METHODS
Participants and grouping
This investigation has recruited 120 male adults (60 positive and 60 control, age from 18-65) who participated in Third People’s Hospital of Huzhou researching program (2015/7-2016/7). Collected data include social-demographic measurements; character of personality, temperament, and psychopathology; general social and cognitive functioning; health-impairing attributes of habit and lifestyle; biological measurements of serum makers; and DNA for the study of genetic variation and phenotypes. All included participants fulfilled ICD-10 diagnosis standard.
Exclusions from our participation including: history of chronic kidney or liver disease, atherosclerotic cardiovascular disease, prior years cancer treatment, and major neurological disorders, schizophrenia or other chronic inflammation related illness such as smoking. Other exclusions included pregnancy and medicine history, such as: insulin, glucocorticoid, antiarrhythmic, psychotropic, or prescription weight-loss medications.
Data collections and experimental protocols were approved under guideline of the Ethics Committee of Third People’s Hospital of Huzhou (IBM No. 2015-(6)). 60 depression male patients who has at least one family member had depression symptom were assigned into family-DEP group (fDEP); in contrast, 60 male healthy volunteers were assigned into control group.
Procedure
Prior to laboratory visit, participants were asked to take a 8 hours fast and avoid exercise for 12 h. Participants were also ask to avoid alcohol intake for 24 h. Laboratory samples were all collected in the morning. Upon subjects’ arrival, they were required to complete a brief medical history interview with the help of nurse. After this interview, participants were exam and calculated for BMI (kg/m2) and then drawn 40 mL blood sample for later use. Those collected blood samples was separated into 2 portions. The first part will be used for CRP level detection. The second portion will be used for DNA isolation and PCR exam later.
Variable measures
Circulating CRP
Plasma CRP was measured by using the Abbott Architect c16000 chemistry analyzer (Diamond Diagnostics, MA, USA) as previously described [10]. In this procedure, polystyrene beads were pre-coated with monoclonal antibodies against CRP, which can aggregate CRP antigen and thereby increase measurement sensitivity. This method could effectively detect serum CRP in range of 0.175-1100 mg/L. Logarithmic transformation was used to normalize CRP values distribution.
Depressive symptoms
Depressive symptoms were measured by using Hamilton Rating Scale for Depression (HAMD-17) form. Patients were evaluated and scored by a clinician according to HAMD-17 guideline for 17 aspects. A final total score of 0-7 is considered to be normal. Scores of 18 or higher indicate moderate or severe depression.
DNA isolation and SNPs analysis
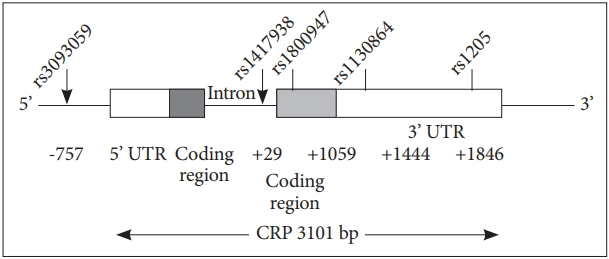
DNA was isolated from frozen whole blood samples as previously described [11,12]. The CRP gene is located on chromosome 1 (1q21-23), which has two exons and encodes a protein with 204 amino acid length. In the present study, we have researched on five common CRP gene polymorphisms: rs3093059, rs1417938, rs1800947, rs1130864 and rs1205 (Figure 1). We selected these markers by using Tagger algorithm and the HapMap genotype data in China [13]. Subjects were genotyped by using high resolution melting method (LightCycler 480; Roche, ShangHai, China) followed by software analyze (LightCycler 480 software release 1.5.1.62 SP1; Roche).
Statistical analyses
All data statistical analyses were performed by using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). χ2-tests with Bonferroni correction was used to compare CRP genotypes distribution with those predicted. The association between CRP SNPs and circulating CRP concentration was investigated and evaluated by using one-way ANOVA with tukey post hoc. The Data were expressed as mean±SEM.
RESULTS
Population characteristics
Table 1 shows demographic, biochemical and inflammatory values in fDEP and control groups. No statistically significant differences (p<0.05) was found other than circulating CRP for all the parameters that we collected here.
Circulating CRP levels and depression
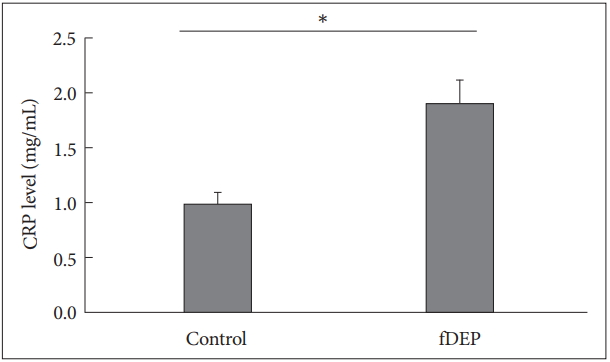
In our research, we have found fDEP patients have a significant higher circulating CRP level than control patients. In control group, the circulating CRP level was 1.871±0.152 mg/L, while in fDEP patients, this number was increased to 2.811±0.341 mg/L (p=0.014) (Figure 2).
CRP polymorphisms distribution in fDEP
Here we involved 5 well established CRP SNPs (rs3093059, rs1417938, rs1800947, rs1130864 and rs1205) (Figure 1) polymorphisms and found A allele in rs1417938, G allele in rs1800947 and C allele in rs1205 gene distribution were significantly increased in fDEP patients, while rs3093059 and rs1130864 did not have major distribution difference between fDEP and controls (Table 2).
CRP polymorphism and circulation CRP level
The results here showed participants with rs3093059 and rs1130864 SNPs have comparable serum CRP level. However, we found compare with its major allele, both CC allele in rs1205 and AA allele in rs1417938 SNP could lead to a significant higher serum CRP level. In contrast, participants with CC allele in rs1800947 showed a decreased trend of CRP level compare with major alleles (GG) (Table 3).
DISCUSSION
In the present study, we have researched multiple demographic, biochemical and inflammatory values between fDEP patients and healthy controls. As shown in Table 1, most of those factors were not significantly changed between these two groups. However, as shown in Figure 2, we have found an increase in serum CRP level in fDEP patients. Furthermore, we found that two of five variants of the CRP gene SNPs (compare with its major allele) were modest but significantly associated with patients who had a positive family depression history. Interestingly, those SNPs appeared could up-regulate serum CRP level, which also consistent with previously reports that elevated serum CRP may lead to depression.
To date, inconsistent results have been reported from studies working on CRP variants and depression. Two studies have found no associations between depressive and serum CRP level [14,15]. In contrast, Luciano et al. [16] group examined the relations between depressive mood state and CRP SNPs in two elderly cohorts while adjusting for age and gender. They reported a modest significant association between rs1800947 and depression in one of the cohorts. Although they found there was no association between depression and rs1205, another [17] group reported an association between rs1205 and depression in 3700 men aged 70 years and over. One more research also indicated certain CRP gene haplotype was positively related with depression and high serum CRP level [18].
Considering the difference between reports may come from individual patients variations, the current viewpoint in literatures is some certain CRP SNPs could regulate serum CRP level [19-23]. For example, CC allele on rs1205 could up regulate serum CRP level [24,25]. In the present study, we have also found CC alleles in rs1205 and AA alleles in rs1417938 showed a significant higher serum CRP level, meanwhile CC alleles in rs1800947 showed a decrease of serum CRP compare with its major alleles (GG). The detailed mechanisms for those regulations may come from gene epigenetic modifications, such as nucleotide methylation caused by different SNP polymorphisms in gene regulatory regions.
Because both depression and CRP polymorphism are around 40% inheritable, we thus analyzed our data and tried to find out whether CRP polymorphism might related to depression in an inherited manner. Our results here showed that A allele in rs1417938, G allele in rs1800947 and C allele in rs1205 gene were potentially related with fDEP patients. Those data indicated some certain CRP polymorphisms were positive correlated with family inherited depression. Considering inherited factors would usually show dominant effects than other sporadic factors in diseases, our data strongly support the idea that CRP polymorphisms may positively associated with depression occurrence.
In summary, by researching inherited CRP SNPs and depression, our data have showed fDEP patients had higher serum CRP level than control, which indicated higher serum CRP level might positively related with depression. Secondly, our data showed some certain CRP SNPs distributed in fDEP patients with higher frequency, which strongly proved those CRP polymorphisms were positively associated with depression. Finally, we have found 2 SNPs which distributed more in depression patients could up-regulate serum CRP level. All of our data together showed family depression is related with inherited higher serum CRP level, which also strongly indicated higher serum CRP level may lead to depression occurrence.