 |
 |
- Search
| Psychiatry Investig > Volume 17(10); 2020 > Article |
|
Abstract
Objective
Methods
ACKNOWLEDGEMENTS
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Hayun Choi, Kiwon Kim, In-Young Yoon. Data curation: EunYoung Lee. Formal analysis: EunYoung Lee, Hayun Choi. Funding acquisition: Hayun Choi. Investigation: EunYoung Lee. Methodology: EunYoung Lee, Hayun Choi. Project administration: EunYoung Lee, Hayun Choi. Resources: Hayun Choi, Kiwon Kim, Hyung Seok So, Jin Hee Choi. Software: EunYoung Lee, Hayun Choi. Supervision: Hayun Choi, In-Young Yoon. Validation: EunYoung Lee. Visualization: EunYoung Lee, Hayun Choi. Writing—original draft: EunYoung Lee. Writing—review & editing: Hayun Choi, Kiwon Kim, Hyung Seok So.
Figure 1.
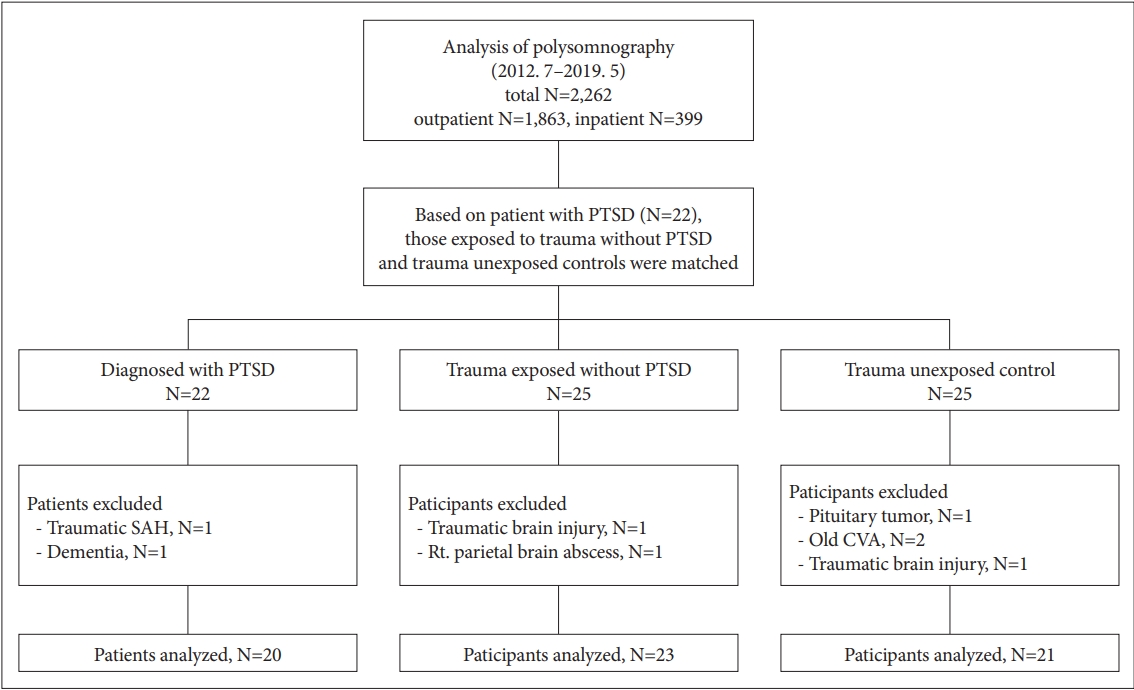
Table 1.
| PTSD (N=20) | Trauma-exposed (N=23) | Control (N=21) | p | |
|---|---|---|---|---|
| Age, yr | 67.85 (8.54)* | 63.96 (13.35) | 59.86 (10.90) | 0.036 |
| BMI, kg/m2 | 24.66 (2.98) | 25.89 (3.73) | 25.71 (3.33) | 0.454 |
| Education | 11.74 (3.00) | 13.27 (2.33) | 12.40 (3.63) | 0.29 |
| PSQI | 9.37 (3.77) | 9.78(4.10) | 7.11 (4.12) | 0.085 |
| SSS | 3.42 (1.46)* | 3.05 (1.17) | 2.25 (1.16) | 0.005 |
| ESS | 7.63 (5.08) | 10.48 (5.43)* | 6.67 (4.90) | 0.045 |
| ISI | 13.26 (6.68)* | 11.39 (6.65) | 6.40 (7.32) | 0.003 |
| DBAS | 6.39 (1.85)* | 5.59 (1.74) | 4.52 (1.74) | 0.007 |
| BDI | 19.68 (11.91)* | 12.83 (8.61)* | 7.05 (6.13) | 0.001 |
| Cormobidity | ||||
| Cardiovascular ds | 11 (57.9) | 18 (78.3) | 11 (55.0) | 0.217 |
| Neurologic ds | 4 (21.1) | 2 (8.7) | 1 (5.0) | 0.315 |
| Pulmonary ds | 3 (15.8) | 2 (8.7) | 1 (5.0) | 0.575 |
| Gastrointestinal ds | 7 (36.8) | 10 (43.5) | 4 (20.0) | 0.254 |
| Endocrine ds | 8 (42.1) | 13 (56.5) | 4 (20.0) | 0.051 |
| Urological ds | 13 (68.4) | 14 (60.9) | 9 (45.5) | 0.315 |
| Other psychiatric ds | 15 (78.9) | 4 (17.4) | 1 (5.0) | <0.001 |
| Medication | ||||
| Antidepressants | 16 (80.0) | 4 (17.4) | 2 (9.5) | <0.001 |
| Benzodiazepines | 14 (70.0) | 4 (17.4) | 3 (14.3) | <0.001 |
| Antipsychotics | 9 (45.0) | 0 (0.0) | 0 (0.0) | <0.001 |
| Reason for PSG | 0.006 | |||
| Dream enactment behavior | 11 (55.0) | 4 (17.4) | 5 (23.8) | |
| Snoring/apnea | 6 (30.0) | 19 (82.6) | 15 (71.4) | |
| Others | 3 (15.0) | 0 (0.0) | 1 (6.3) |
PTSD: posttraumatic stress disorder, BMI: Body Mass Index, PSQI: Pittsburgh Sleep Quality Index, SSS: Stanford Sleepiness Scale, ESS: Epworth Sleepiness Scale, ISI: Insomnia Severity Index, DBAS: Korean version-Dysfunctional Beliefs and Attitudes about Sleep Questionnaire-16, K-DBAS-16, BDI: Beck Depression Inventory-II, ds: disease, PSG: polysomnography
Table 2.
| PTSD (N=20) | Trauma-exposed (N=23) | Control (N=21) | p | |
|---|---|---|---|---|
| TST, min | 298.11 (60.67) | 304.94 (72.07) | 315.28 (52.93) | 0.685 |
| WASO, min | 91.91 (55.64) | 103.73 (77.86) | 74.46 (52.54) | 0.318 |
| Sleep latency, min | 39.15 (52.13) | 21.72 (35.36) | 16.31 (17.35) | 0.349 |
| Sleep efficiency, % | 70.42 (16.40) | 71.32 (17.74) | 77.98 (13.61) | 0.323 |
| Stage 1, % | 15.86 (8.64) | 16.29 (9.95) | 15.77 (9.96) | 0.972 |
| Stage 2, % | 58.91 (16.71) | 53.54 (13.27) | 59.43 (10.23) | 0.288 |
| Stage 3, % | 4.75 (9.47) | 9.58 (12.88) | 6.67 (6.79) | 0.159 |
| REM, % | 20.48 (10.82) | 20.57 (9.69) | 18.14 (8.20) | 0.648 |
| AHI | 22.45 (22.71) | 32.71 (22.17) | 36.32 (25.43) | 0.067 |
| NRAHII | 22.21 (23.63) | 34.72 (25.94) | 36.41 (26.56) | 0.102 |
| RAHII | 22.34 (18.57) | 24.67 (13.78) | 37.87 (28.04) | 0.158 |
| ODI, events/hr | 8.97 (10.93)* | 15.38 (10.21) | 20.75 (16.71) | 0.019 |
| SaO2<90, % | 4.95 (9.35) | 5.24 (6.44) | 9.33 (14.42) | 0.154 |
| MinSaO2, % | 83.75 (6.89) | 82.48 (5.83) | 79.71 (7.55) | 0.154 |
| PLM Ar | 4.43 (11.44) | 1.29 (3.99) | 1.89 (3.59) | 0.373 |
| PLMI | 18.25 (26.09) | 8.20 (17.91) | 16.92 (30.78) | 0.441 |
| RSWA | 9 (45.0)† | 2 (8.7) | 6 (28.6) | 0.026 |
TST: total sleep time, WASO: wake after sleep onset, REM: rapid eye movement, AHI: apnea-hypopnea index, NRAHI: non rapie eye movement apnea-hypopnea index, RAHI: rapid eye movement apnea-hypopnea index, ODI: oxygen desaturation index, SaO2: oxygen saturation, PLM Ar: periodic limb movements with arousal, PLMI: periodic limb movement index, RSWA: rapid eye movement sleep without atonia
Table 3.
| Outcome | Predictor |
Simple |
Multiple |
||
|---|---|---|---|---|---|
| OR (95% Cl) | p | OR (95% Cl) | p | ||
| RBD | PTSD group* | 14.667 (1.634-131.657) | 0.016 | 11.101 (1.004-122.771) | 0.049 |
| Control group* | 5.176 (0.529-50.653) | 0.158 | 12.921 (0.965-172.953) | 0.053 | |
| Age | 1.186 (1.041-1.352) | 0.010 | 1.234 (1.041-1.464) | 0.015 | |
| AHI | 0.935 (0.886-0.935) | 0.010 | 0.960 (0.916-1.006) | 0.089 | |
| ODI | 0.901 (0.827-0.981) | 0.016 | |||
| BDI | 1.014 (0.957-1.074) | 0.639 | |||
| Neurologic ds | 3.375 (0.651-17.509) | 0.148 | |||
| Other psychiatric ds | 3.231 (0.915-11.406) | 0.068 | |||
| Antidepressants | 0.167 (0.806-9.731) | 0.105 | |||
| Benzodiazepines | 3.083 (0.882-10.782) | 0.078 | |||
| Antipsychotics | 2.200 (0.0469-10.331) | 0.318 | |||
REFERENCES







