Elevated Serum Galectin-1 and Galectin-3 Levels in Children With Specific Learning Disorder: A Case Control Study
Article information
Abstract
Objective
Specific learning disorder (SLD) is a neurodevelopmental disorder in which underlying pathogenesis and etiological factors are not fully understood. Neuroinflammatory response (measured with serum levels of galectin-1 and galectin-3), which is associated with learning and memory, may play an important role in the etiopathogenesis of SLD. Aim of the present study is to examine whether serum galectin-1 and galectin-3 levels are related to SLD.
Methods
The current study consisted of 42 treatment-naive children with SLD and 42 control subjects. All of the subjects were assessed using semi-structured psychiatric examination to diagnose SLD and exclude attention-deficit hyperactivity disorder. Serum galectin-1 and galectin-3 levels were measured via venous blood samples.
Results
The SLD and control group did not differ significantly in terms of age, sex, and body mass index (BMI). The SLD group had significantly higher serum levels of galectin-1 (8.78±2.97 vs. 7.40±2.03, p=0.019) and galectin-3 (1.86±0.93 vs. 1.32±0.69, p=0.003) than the control group when controlled for age, sex, and BMI.
Conclusion
Higher serum levels of galectin-1 and galectin-3 in children with SLD may indicate the role of neuroinflammatory response in the pathogenesis of SLD. Other mechanisms involving galectin-1 and galectin-3 related to learning may play a part in the etiology of SLD.
INTRODUCTION
Specific learning disorder (SLD) is a neurodevelopmental disorder with impairments in learning, reading, and writing skills despite intervention at least six months. The prevalence of SLD is around 5%–15% among school-age children [1]. Although the etiology of SLD is still unclear, it is suggested that genetic, environmental, psychosocial, and neurobiological factors play a role in its development [2]. Recent studies revealed significant links between neuroinflammation and immune system dysfunction with the pathophysiology of neurodevelopmental disorders such as attention-deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) [3-5]. SLD has a common etiological origin with disorders such as ADHD and ASD. Recent studies examined biomarkers of SLD [6-8], whereas no particular attention was given to neuroinflammatory biomarkers.
Neuroinflammation is a fundamental process for facilitating homeostasis and regeneration in the central nervous system (CNS). It is mainly carried out by microglia cells and its main function is to limit the inflammation of neural tissue and eliminate pathogens, cellular debris, and misfolded proteins [9]. Although neuroinflammation is a homeostatic mechanism, when it becomes chronic, it damages neuronal cells [4]. Neuroinflammation is suggested to increase the risk of neurodevelopmental disorders through glial activation [10], increased oxidative stress [11], aberrant neuronal development [12], reduced neurotropic support [13], and altered neurotransmitter function [14]. Studies to date have revealed a link between neuroinflammation and cognitive impairments such as working memory and visuospatial memory problems, which are commonly observed in individuals with SLD [15-18]. However, the potential role of persistent inflammation in the CNS has not been adequately studied in the etiopathogenesis of SLD.
In recent years, galectins were given a particular focus in studies examining the role of neuroinflammation in the etiopathogenesis of neurodegenerative diseases and neurodevelopmental disorders. Galectin-1 and galectin-3 are the most widely studied galectin proteins [19]. Galectin-1 is expressed in skeletal and cardiac muscle cells (myocytes), hepatocytes, and prostate cells, whereas galectin-3 is expressed in epithelial cells of the digestive and respiratory tracts [20]. Galectin-1 is an important modulator in maintaining homeostasis in the CNS and may play a role in neuroinflammation or neuroprotective processes depending on its environment [21]. Several studies have reported that galectin-1 expression is altered in neurological diseases [22,23]. On the other hand, galectin-3 takes part in various physiological processes such as cell adhesion, cell activation, cell growth and differentiation, cell cycle and apoptosis, as well as cell-cell interactions [24,25]. Galectin-3 is also found in various immune cells other than resting lymphocytes and acts as a pro-inflammatory mediator in inflammation [24,26]. In addition, galectin-3 is one of the important initiators of microglia activation and proliferation following CNS damage, and the interaction between galectin-3 and toll-like receptor 2 on microglia is necessary to initiate neuroinflammation [27-29]. Generally, galectin-3 plays a key role as a proinflammatory factor in neuroinflammatory and neurodegenerative processes in the CNS, and it may be a potential biomarker in the detection and evaluation of disease progression [19].
As the association between galectin-1 and galectin-3 levels and CNS diseases is established, interest in the relationship between galectins and psychiatric disorders has increased in recent years. For example, recent studies documented higher galectin-3 levels in patients with schizophrenia compared to healthy controls [30,31]. Also, galectin-1 levels were higher in the unaffected siblings of patients with schizophrenia than both the patient group and healthy controls [32]. Galectin-3 levels among those with ADHD were found to be lower in one study whereas they were higher in another recent study, indicating mixed findings [33]. Although there is no clinical research on galectin-1 levels in ADHD, some animal studies documented the effect of galectin-1 on learning and memory. For example, galectin-1 gene knockout mice showed impairments in spatial learning and memory, leading to the conclusion that galectin-1 plays a key role in hippocampal learning and memory [18]. In addition, mice with chronic stress-related depressive features had higher serum galectin-1 levels, suggesting galectin-1 levels may increase in both the CNS and peripheral tissues in response to biological stress [34].
ADHD comorbidity and increased depressive symptoms are frequently observed among individuals with SLD [35]. In addition, recent research showed that neurodegenerative diseases associated with galectin-1 and galectin-3 are more common among those with SLD [36]. Finally, studies to date documented the relationship between galectin-1 and galectin-3 levels with the presence of cognitive impairments such as problems with hippocampal learning, executive functions, and spatial memory, which are the hallmarks of SLD [15,18]. Hence, further research on neuroinflammation in individuals with SLD may shed light on both the etiology of SLD and potential mechanisms for the increased risk of neurodegenerative diseases among those with SLD. The present study examined the neuroinflammatory markers serum galectin-1 and galectin-3 levels in children with SLD without ADHD comorbidity and compared them with healthy controls. We hypothesize that serum galectin-1 and galectin-3 levels will be higher among SLD cases compared to healthy controls.
METHODS
Participants
Participants of the case group were recruited from the outpatient clinic of the Department of Child and Adolescent Psychiatry at the Istanbul Faculty of Medicine, Istanbul University. The case group consisted of children with SLD diagnosis according to Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) [1] criteria. The control group consisted of age-gender matched children who were referred to the outpatient clinics of the pediatrics department at the Istanbul Faculty of Medicine for routine checkups. Exclusion criteria for both groups are as follows: presence of a major medical comorbidity (e.g., metabolic/genetic/autoimmune diseases, neurological disorders, visual or hearing impairments, etc.), schizophrenia, bipolar disorder, ADHD, ASD and/or an intelligence quotient (IQ) score below 80 according to the Turkish version of the Wechsler Intelligence Scale for Children-Revised (WISC-R), and psychotropic medication use in the last year [37].
The children with SLD were diagnosed by child and adolescent psychiatrists based on DSM-5 criteria. In addition to DSM–based clinical evaluations, SLD clinical observation battery (SLD-COB) was used to determine the problematic learning areas of the children with SLD diagnoses [38,39]. According to the SLD-COB, 59.5% (n=25) of children have specific impairments in reading, written expression, and mathematics; 31.0% (n=13) had specified with impairments only in reading and written expression; and 9.5% (n=4) had specified impairments only in reading. WISC-R was also performed for all participants to detect any intellectual disabilities. The assessment and evaluation of these tests were conducted by psychologists with specific training and experience in the application of the tests. Child and adolescent psychiatrists in the research team evaluated all participants for psychiatric disorders using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version–DSM-5 (K-SADS-PL-DSM-5). The parents also completed Conners’ Parent Rating Scale–Revised Short (CPRS-RS) and a sociodemographic form developed by the researchers.
Among 61 children in the case group, 17 were excluded from the study based on the exclusion criteria (7 were diagnosed with ADHD, 1 was diagnosed with ASD according to the K-SADS-PL, SLD diagnoses of 2 were not supported by the SLD-COB, and 7 were total IQ scores of below 80 when screened with WISC-R) and 2 refused to give blood samples following the evaluation. The final case group consisted of 42 children with SLD. The control group consisted of children who referred to the outpatient clinics of the pediatrics at the Istanbul Faculty of Medicine for routine checkups. Psychometric and psychiatric evaluations performed for the control group were the same as those of the case group. Among a total number of 57 children, 12 were excluded from the study (6 were diagnosed with ADHD according to the K-SADS-PL, 2 were diagnosed with SLD according to DSM-5 criteria and SLD-COB, and 4 were total IQ scores of below 80 when screened with WISC-R) and 3 refused to give blood samples following the evaluations. The final control group consisted of 42 neurotypical children (Figure 1).
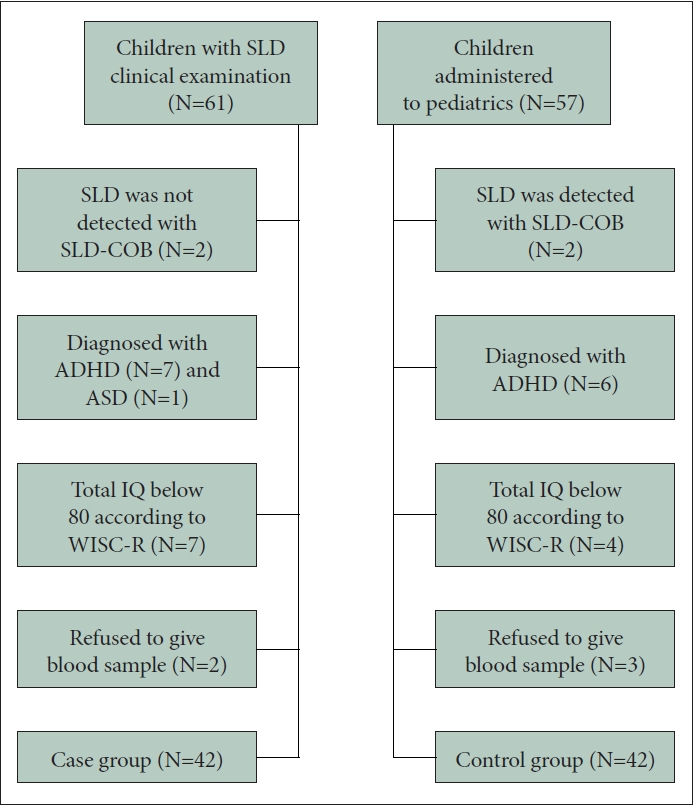
Flow diagram of the study. SLD, specific learning disorder; SLD-COB, SLD clinical observation battery; ADHD, attentiondeficit hyperactivity disorder; ASD, autism spectrum disorder; IQ, intelligence quotient; WISC-R, Wechsler Intelligence Scale for Children-Revised.
All participants gave the verbal assent and their parents gave the written informed consent. The study was approved by the Istanbul University, Istanbul Faculty of Medicine Clinical Research Ethics Committee (date: 09/09/2020, no: 29624016-050.99-1307) and all procedures were in accordance with the standards in the Declaration of Helsinki.
Measures
SLD-COB
The battery was initially developed by Korkmazlar [38] and then was revised to involve new subscales. Nine subscales which assess reading/writing/arithmetic skills, Gessell figures, ability to draw a clock, right–left discrimination, lateralization, before–after relationships, and ordering are included into the battery. SLD-COB was used to determine the specific type of learning disorders (dyslexia, dysgraphia, and dyscalculia).
K-SADS-PL-DSM-5
The K-SADS-PL is a semi-structured interview which was initially developed by Chambers et al. [40] to assess the present and lifetime psychopathology in children and adolescents. It was revised according to DSM-III and DSM-IV criteria. Turkish adaptation of the updated version of K-SADS-PL according to DSM-5 criteria was conducted by Ünal et al. [41].
CPRS-RS
This 27-item, 4-point Likert-type scale assesses ADHD symptoms and other behavioral problems in children and adolescents. Inattention/cognitive problems, hyperactivity, and oppositional behavior subscales are included in CPRS-RS. The reliability and the validity of the Turkish adaptation was established by Kaner et al. [42].
WISC-R
This scale was established to determine IQ levels of children aged between 6–16 years. Originally, it was developed by Wechsler as the Wechsler-Bellevue scale to assess adults which then was revised for children in 1949. WISC-R is a revised version of WISC in 1974 [43]. Turkish adaptation of the WISC-R was used in the present study [37]. The results of the scale consists of verbal IQ, performance IQ, and full–scale IQ.
Blood sampling
Venous blood samples of the patients and controls were collected between 8:00 and 10:00 Am to avoid circadian variation. Participants were also informed to abstain from heavy exercise, eating, and drinking prior to sampling. Blood samples were centrifuged, and the serum specimens were stored at -80°C until the analysis. Serum levels of galectin-3 and galectin-1 were measured by the enzyme–linked immunosorbent assay (ELISA) method using commercial human ELISA test kits and in accordance with the protocols of manufacturers (Elabscience Biotechnology Inc., Texas, USA).
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics 21.0 package program (IBM Corp. Released 2012, Armonk, NY, USA). Data were presented in the form of counts, frequencies, median (25–75 percentiles), and mean±standard deviation. Categorical variables between groups were compared by chi-square test. For non-normally distributed continuous variables, Mann–Whitney U test was used to compare groups. For normally distributed data, the Student’s t-test was performed. The correlation between continuous variables was examined by Spearman’s rank correlation analysis. Analysis of covariance (ANCOVA) was used to compare galectin 1 and 3 levels between case and control groups, where age, sex, and body mass index (BMI) were the covariates. The significance threshold was set at p<0.05.
RESULTS
There was no significant difference between the SLD and control group regarding age, sex, and BMI (Table 1).

The sociodemographic characteristics of children with specific learning disorder (SLD) and the control group
The rate of children having at least one comorbid psychiatric disorder was 38.1% in the SLD group and 33.3% in the control group (χ2=0.27, p=0.65). The most common comorbidities in the SLD group were specific phobias (23.8%), social anxiety disorder (9.5%), and enuresis (7.1%); and those in the control group were specific phobias (19.0%), generalized anxiety disorder (4.8%), and tic disorders (4.8%). Verbal, performance, and full–scale IQ scores were lower in the SLD group than the control group. The inattention/cognitive problems’ subscale scores of CPRS-RS were higher in the case group than the control group (z=-4.951, p<0.001) (Table 1).
Serum galectin 1 and 3 levels of the case group were significantly higher than the control group according to the ANCOVA test where age, sex, and BMI were the covariates (Table 2 and Figure 2).
DISCUSSION
In the current study, serum galectin-1 and galectin-3 levels were compared between children with SLD and healthy controls. The results showed that the children with SLD had higher serum levels of galectin-1 and galectin-3 than controls, controlling for gender, age, and BMI. The findings of this cross-sectional study suggest that galectin-1 and galectin-3 may be a factor in the etiopathogenesis of SLD.
One of the major findings of the current study is that serum galectin-1 levels were higher among the children with SLD compared to healthy controls. The etiological role of galectin-1 on learning is not yet fully understood. To date, a single mice study investigated the direct effect of galectin-1 on learning. In that study, learning and memory processes were significantly impaired in galectin-1 gene knockout mice, but motor and sensory skills remained intact [18]. SLD is characterized by marked impairment in learning and memory processes [44]. Decreased serum levels of galectin-1 may be in accordance with the previous study in mice. However, in the present study, galectin-1 levels were higher than the control group which might be a compensatory response to enhance learning. Future studies with detailed neuropsychological evaluations may shed light on the effects of galectin-1 levels on learning processes in human.
An important finding of the current study is higher serum galectin-3 levels in children with SLD compared to healthy controls. Although, the direct effects of galectin-3 on learning were not clearly understood, it was shown in various studies that galectin-3 impact learning and memory through neuroinflammatory processes [45,46]. Firstly, in an animal study, it was shown that the memory of rats was impaired as a result of neuroinflammation induced by amyloid-β 25–35 administration, and this effect was linked to increased microglial galectin-3 expression in dorsal hippocampus [46]. Additionally, decreased galectin-3 expression had a positive effect on learning and memory [47]. The findings of the current study were in line with the existing literature, further research concerning the clinical significance of elevated serum galectin-3 levels in human subjects is needed.
Higher serum levels of galectin-1 and galectin-3 in children with SLD suggest that neuroinflammation may have a role in the development of SLD. Neuroinflammation is supposed to increase the risk of neurodevelopmental disorders through processes such as glial activation and increased oxidative stress [10,11]. It is known that both galectin-1 and galectin-3 are key agents in neuroinflammation [48]. Galectin-1 is a neuromodulator with anti-inflammatory effects, whereas galectin-3 is reported to have both pro- and anti-inflammatory effects. It is suggested that galectin-3 promotes inflammatory response by suppressing the production of the anti-inflammatory cytokine interleukin-10 (IL-10). An in vitro study showed that galectin-3 induced IL-6 expression, which decreased galectin-3 inhibition. To our knowledge, no prior study examined neuroinflammation in individuals with SLD, whereas one study to date reported increased peripheral inflammation [8]. The current study is the first to focus on neuroinflammation in children with SLD, and our findings indicate that further research is needed on this topic.
Galectin-1 and galectin-3 were studied as a biomarkers in different psychiatric disorders such as schizophrenia, ASD, and ADHD. Serum levels of galectin-3 were higher in subjects with schizophrenia than control group in a study, whereas another study reported that serum levels of galectin-3 were lower in the first-episode and relapsed patients with schizophrenia, and higher in patients in remission, compared to healthy control group [30,32]. In another study, serum levels of galectin-1 and galectin-3 were higher in children with ASD than healthy controls [49]. Additionally, serum galectin-3 levels were reported to be lower in children with ADHD than the control group [33]. The findings of the present study and the studies with other neurodevelopmental disorders may be interpreted as an increase in serum levels of galectin-1 and galectin-3 may be a response to chronic inflammation [32]. Schizophrenia, ASD, and ADHD are frequently accompanied by learning problems [50]. Hence, the findings of the current study indicate a need for further research on the effects of neuroinflammation on learning processes.
The findings of the present study should be interpreted taking some limitations into consideration. First, since the design was cross-sectional, a causal relationship between serum galectin 1 and 3 levels and SLD can not be inferred from these findings. Secondly, the sample size was relatively small due to exclusion of some participants through psychiatric examinations and intelligence tests, which limits the statistical power of the study. Third, although the sample was recruited from a central reference clinic, recruiting cases from a single center may prevents the generalizability of our findings to all SLD patients. Fourth, although the role of galectin 1 and 3 in neuroinflammation is well-established in the literature, since their link with peripheral inflammatory response was not demonstrated using different biomarkers in the current sample, inferences about inflammation would be limited. Fifthly, neuropsychological characteristics of the sample were not investigated. Finally, although the diagnosis of ADHD was among the exclusion criteria, some of the cases had comorbid psychiatric disorders accompanying SLD. Despite these limitations, there are also several strengths of the present study; SLD was diagnosed by clinical evaluation and supported by intelligence test and SLD-COB, children with comorbid ADHD were detected via semi-structured diagnostic interview and were excluded, and all subjects were drug-naive.
In conclusion, the present study is the first case-control study in which serum levels of galectin-1 and galectin-3, which were shown to impact learning and memory processes, were studied in children with SLD. Higher levels of galectin-1 and galectin-3 in individuals with SLD suggest that neuroinflammatory processes may be a prominent factor in the etiology of the disorder. SLD causes chronic and long-term disability with a high societal cost, which necessitate more detailed and longitudinal studies. Further studies may provide information about the etiology, diagnosis and treatment processes of the disorder.
Notes
Availability of Data and Material
All data generated or analyzed during the study are included in this published article.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Yaşar Tanır. Data curation: Yaşar Tanır, Zeynep Nur Gülle. Formal analysis: Yaşar Tanır, Adile Merve Baki, Pervin Vural. Investigation: Yaşar Tanır, Gökçe Sultan Uncu, Nusret Soylu. Writing—original draft: Yaşar Tanır, Zeynep Nur Gülle, Abdurrahman Cahid Örengül. Writing—review and editing: Yaşar Tanır, Abdurrahman Cahid Örengül.
Funding Statement
Financial support for the study was provided by the Istanbul University Scientific Research Projects Unit [No: 37122].
Acknowledgements
Special thanks are extended to all of the participants.


