 |
 |
- Search
| Psychiatry Investig > Volume 20(11); 2023 > Article |
|
Abstract
Objective
Methods
Results
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are not publicly available because the data include patient information but are available from the corresponding author on reasonable request.
Conflicts of Interest
Yoo Hyun Um and Hyun Kook Lim, a contributing editor of the Psychiatry Investigation, were not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions
Conceptualization: Sheng-Min Wang, Hyun Kook Lim. Data curation: Sheng-Min Wang, Hyun Kook Lim. Formal analysis: Sheng-Min Wang, Hyun Kook Lim. Funding acquisition: Sheng-Min Wang, Hyun Kook Lim. Investigation: Yoo Hyun Um, Dong Woo Kang, Sunghwan Kim. Methodology: Sheng-Min Wang, Hyun Kook Lim, Sunghwan Kim. Project administration: Yoo Hyun Um, Dong Woo Kang, Chang Uk Lee. Resources: Sheng-Min Wang, Sunghwan Kim. Software: Sheng-Min Wang, Sunghwan Kim. Supervision: Hyun Kook Lim, Chang Uk Lee. Validation: Yoo Hyun Um, Dong Woo Kang. Visualization: Chang Uk Lee, Yoo Hyun Um. Writing— original draft: Sheng-Min Wang. Writing—review & editing: Sheng- Min Wang, Hyun Kook Lim.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2022R1A2C1093215). This research was also supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) and Korea Dementia Research Center (KDRC), funded by the Ministry of Health & Welfare and Ministry of Science and ICT, Republic of Korea (grant number: HU20C0315).
Figure 1.
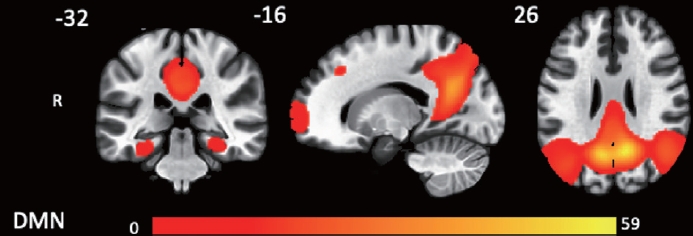
Figure 2.
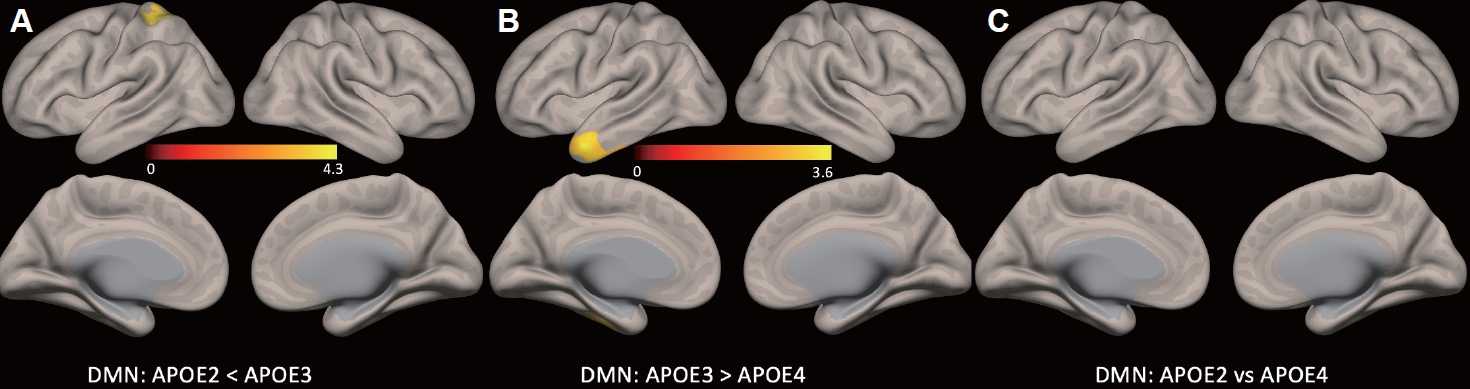
Table 1.
Values are presented as mean±standard deviation or number. A-PEN, amyloid positron emission tomography; CN, cognitive normal older adults; APOE, Apolipoprotein E; M, male; F, female; SUVR, standardized uptake value ratio; CERAD-K, The Korean Version of Consortium to Establish A Registry For Alzheimer’s Disease; VF, Verbal Fluency; BNT, 15-Item Boston Naming Test; MMSE, Mini Mental Status Examination; WLM, Word List Memory; CP, Constructional Praxis; WLR, Word List Recall; WLRc, Word List Recognition; CR, Constructional Recall; NS, not significant
Table 2.
| Region | L/R | Cluster | T score | p* | MNI (x, y, z) |
|---|---|---|---|---|---|
| Group differences | |||||
| APOE 3/3>APOE 2/2 or 2/3 | |||||
| Left superior lobe | L | 238 | 4.14 | <0.05 | -26, -40, +58 |
| APOE 3/3>APOE 3/4 or 4/4 | |||||
| Temporal lobe | L | 338 | 3.46 | <0.05 | -48, +08, -36 |
| APOE 2/2 or 2/3 vs. APOE 3/4 or 4/4 | |||||
| No significant differences |
REFERENCES







