 |
 |
- Search
| Psychiatry Investig > Volume 20(12); 2023 > Article |
|
Abstract
Objective
Believing that oxidative stress may be increased in borderline personality disorder (BPD) patients with self-mutilating behaviors (SMB), we aimed to measure serum asymmetric dimethylarginine (ADMA) and malondialdehyde (MDA) levels in these patients.
Methods
The study included 60 patients diagnosed with BPD and 30 healthy controls. BPD patients were divided into two groups: 30 female patients with SMB and 30 female patients with no-self-mutilating behavior (NSMB). ADMA, MDA, vitamin A, and vitamin E levels were analyzed. Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI) were conducted with the participants.
Results
Serum ADMA and MDA levels were higher in SMB and NSMB group compared to control group. Serum vitamin E levels were also lower in the SMB group compared to the control group. Positive correlations were determined between both ADMA and MDA, and between BDI and BAI scores. Also, BAI scores were statistically higher in SMB group compared to NSMB group.
Conclusion
It was discovered that levels of ADMA and MDA, which reflected oxidative stress, were elevated in patients with BPD who exhibited SMB. Accordingly, future studies should investigate the role of oxidative stress in a more comprehensive way in terms of the different mechanisms underlying and treatments involved in borderline personality disorder.
Borderline personality disorder (BPD) is a behavioral condition that is persistent and impulsive, affecting 2%-3% of the population [1]. In Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) of the American Psychiatric Association [2], the main characteristics of BPD were reported as a pervasive model of instability in interpersonal relations, self-image, and marked impulsivity, which is initiated in early adulthood and exhibited in various contexts. It was also reported that BPD had a significantly elevated comorbidity with other psychiatric conditions, such as general anxiety, clinic depression, and post-traumatic stress disorder such that more than 70% of individuals with BPD also met the criteria for at least 3 other psychiatric conditions [3].
The different mechanisms underlying and pathophysiology of BPD have not been investigated comprehensively. It was reported that stress and dysphoria were among the common experiences in individuals who were diagnosed with BPD [4]. It was also stated that social stressor factors that are related to personality disorders, such as social defeat, maternal, and social deprivations, were among the factors that exacerbated oxidative stress [5]. Inflammation and chronic stress are thought to have a major role in the pathogenesis of BPD and many psychiatric disorders [6]. BPD is more common in women because of the high prevalence rate of sexual abuse and such life stressors in women [7]. Anger that is not regulated and the manifestations of anger that are reflected in behavior, such as physical violence, were also reported as criteria that define BPD. Furthermore, it was stated that the patients with BPD were at significantly high risk in terms of attempts and committing suicide [8]. Moreover, significant pieces of evidence demonstrated that implicitly excessive nitric oxide (NO) or unusual generation of NO were observed in certain neurological and psychiatric disorders. NO was defined as a gaseous neurotransmitter, which is synthesized from L-arginine by a family of enzymes that are recognized as nitric oxide synthase (NOS) [9]. Additionally, NO is formed by neuronal NOS and is very important in the organization of memory and the development of the brain. On the other hand, natural L-arginine analogs, such as asymmetric dimethyl-arginine (ADMA), symmetric dimethyl-arginine, and L-N-monomethyl arginine, could inhibit NO synthesis competitively [10]. It was also reported that ADMA was metabolized by the enzyme, dimethylarginine dimethylaminohydrolase (DDAH) [11]. Recently, oxidative mechanisms have been studied in terms of their role in the pathophysiology of many psychiatric disorders, and it has been suggested that neurotransmitters and amino compounds have an important place here [12].
In the review of the literature, it was discovered that no study investigated ADMA and malondialdehyde (MDA) levels in patients with BPD, which makes the current study a first. Accordingly, the current study aims to evaluate serum ADMA and MDA levels among patients with BPD who exhibit self-mutilating behaviors (SMB) and the patients who do not exhibit self-mutilating behaviors (NSMB).
This study approved by the local ethics committee of Firat University (Approval No: 21.07.2020/11-13). Since the male patients invited to the study did not accept the invitation, 72 female patients who applied to Fırat University Hospital, Mental Health, and Diseases Polyclinic and were diagnosed with BPD, were included in the study. Twelve patients were not included because they did not meet the study criteria or did not want to take place in the study. Consent was obtained from the patients participating in the study and healthy controls. Two groups were formed with 30 female BPD patients with SMB and 30 female BPD patients with NSMB. The Turkish version of the Structured Clinical Interview [13] was used for the diagnosis of BPD. A chart review was also conducted by a psychiatrist (SB).
The inclusion criteria of the sample covered the absence of psychiatric conditions, such as schizophrenia, schizoaffective or bipolar disorder, absence of organic diseases, physical illness, or significant physical pathology, which could impact the distribution of the present psychiatric symptoms as well as no history of alcohol or substance use disorders in the last six months according to DSM-5.
On the other hand, the exclusion criteria of the sample covered the presence of comorbid psychiatric, neurological or medical disorders, which could impact brain function. To minimize the impact of drug administration, the patient group was established with medication-free participants.
A control group of 30 healthy subjects with a similar distribution of sex and age was included in the sample. The control group consisted of individuals who applied to the psychiatry outpatient clinic to receive a report proving that they were psychiatrically healthy for various purposes, such as job applications, and were matched for age in addition to not having any psychiatric, organic, and physical diseases and not using any medication.
Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI) and the SCID-II [13] were applied to the participants. In addition, a sociodemographic data form prepared by us was applied to evaluate the sociodemographic data for all participants. The BDI aims to measure the severity of emotional, motivational, and cognitive symptoms in depression. The inventory includes 21 items evaluated on a 4-point Likert-type scale [14]. The cutoff score is 17 [15]. On the other hand, the BAI is used to determine the frequency of anxiety symptoms in individuals. The inventory is a 3-point Likert-type self-report scale that includes 21 items. On the scale, the scores from 0 to 7 indicate minimal anxiety while 8-15 indicate mild anxiety. Additionally, scores ranging from 16 to 25 indicate moderate anxiety while scores of 26 and above indicate severe anxiety [14].
After an overnight fast, blood samples were taken from the antecubital vein. Blood samples were centrifuged (3,000 rpm, 10 min), and the separated serum and plasma were stored at -80°C until the study day. ADMA levels were measured on high-performance liquid chromatography (HPLC) instrument using commercial kits (EUREKA srl-Laboratory division, Chiarvalle, Italy). The MDA levels were analyzed using an MDA kit (Immuchrom GmbH, Hessen, Germany) with HPLC. Serum vitamin A and vitamin E levels were measured on HPLC instrument by using the ImmuChrom reagent kits (Immuchrom GmbH).
Statistical analyzes were conducted by IBM SPSS version 26.0 (IBM Corp., Armonk, NY, USA). Significant differences among the groups were evaluated by the one-way analysis of variance analysis with the least significant differences test to distinguish the groups. A p-value less than 0.05 was deemed statistically significant. Correlations between the variables were investigated by the Pearson correlation analysis.
Thirty female BPD patients with SMB and 30 female BPD patients with NSMB were included in the study. Also, 30 female healthy control with similar ages were included in the study. The mean age were presented in SMB group, NSMB group, and healty control group (27.80±8.37, 30.06±9.13, 26.20±5.77 years, respectively) (Table 1). Serum vitamin E levels were lower in patients with SMB compared to the control group (p=0.04) (Table 1). However, there was no significant difference in the vitamin A levels in the groups. Furthermore, BAI scores were found to be statistically higher in SMB group compared to NSMB group (p=0.001). Although the BDI scores were higher in the SMB group than in the NSMB group, no statistical difference was found. Laboratory results and BAI and BDI scores of the groups can be found in Table 1. There was a significant difference between the groups in terms of ADMA and MDA levels (p<0.05). ADMA levels were statistically higher in SMB group compared to the control group and NSMB group (p=0.001, p=0.008, respectively) (Table 2). MDA levels were found to be statistically higher in SMB group compared to the control group (p<0.001). Additionally, MDA levels were significantly higher in SMB group compared to NSMB group (p=0.004) (Table 2). In addition, a figure demonstrating ADMA and MDA values in the groups is presented (Figure 1).
Our current study is the first study to measure serum ADMA and MDA levels in patients with BPD, according to the information we know and obtained from our literature review. The results of this study revealed that serum ADMA and MDA levels in BPD patients with SMB were significantly elevated compared to the control and NSMB patient group. Also, MDA levels were significantly higher in SMB group compared to NSMB group. Furthermore, both ADMA and MDA correlated positively with BAI and BDI scores.
ADMA is an endogenous inhibitor of endothelial NOS, which reduces NO levels [11,16]. NO is a neurotransmitter that is observed in high densities in emotion-regulating regions of the brain. Neuronal nitric oxide synthase (nNOS) is mainly expressed in the hippocampus, cortex, hypothalamus, amygdala, dorsal raphe nucleus, and various regions of the brain [17]. Additionally, NO is a neurotransmitter that modulates aggressive and depressive behaviors [18]. In a study, it was reported that acute stress exposure caused increases in NOS expression; therefore, the increase in NO concentration exhibited an effect that was similar to antidepressants in the hippocampus [19]. Nitric oxide/NO synthase (NOS1) genes are have a role in impulsivity and aggression [20]. nNOS was previously linked to rodent behavior by various pharmacologic studies. Similarly, it was reported that the knockdown of the NOS1 gene in mice resulted in changes in behavior, which caused decreases in anxiety and increases in impulsivity aggressiveness [21]. It has been shown that impulsivity and aggressiveness are accompanied in the presence of deletion mutants in the NOS1 gene [22]. The likely involvement of variants of the NOS gene in suicidal behaviors is due to various pieces of evidence. The elevated NOS activity in disrupted sleep was reported among the factors related to depressogenic effects [23]. In a study conducted by Kielstein et al. [24] 2015, it was reported that ADMA infusion resulted in significant decreases in brain-derived neurotropic factor (BDNF) levels, which led to depressive behaviors. In this study, the researchers also reported that there was a negative correlation between BDNF and BDI based on their investigation with rats.
In the current study, we determined that levels of MDA increased in the BPD patients in the SMB group compared with the NSMB group as an indicator of lipid peroxidation, which measures oxidative stress. The increase in reactive oxygen species (ROS) production but also the incompatibility between the antioxidant defense system causes oxidative stress [1]. While oxidative stress can be induced in response to the inflammatory response, as in BPD, oxidative stress can trigger inflammation through the activation of nuclear factor-kappaB (NFkB) [25]. Elevated levels of proinflammatory cytokines are often accompanied by elevated levels of oxidative and nitrosative stress, which have been significantly understudied in BPD. As a result of the change in immune response in BPD, changes occur in the mitochondria of immune cells. Mitochondria are the main source of cellular ROS that occur as a result of increased oxidative stress, and it has been thought that this may be seriously related to BPD [4].
Particularly, the brain is highly susceptible to oxidative damage because it consumes large amounts of oxygen. Also, brain neuron membranes contain highly unsaturated fatty acids, and therefore brain metabolism produces significant amounts of hydrogen peroxide [26]. MDA and 4-hydroxynonenal are among the characteristic and reactive aldehydes and they are end products of lipid peroxidation, which are formed upon the oxidation of polyunsaturated fatty acids [27]. As a result of increased MDA levels in cerebrospinal fluid and plasma, the brain may suffer oxidative damage [28]. Additionally, it was reported that oxidative stress increased ADMA levels via various mechanisms [29]. In terms of oxidative stress, DDAH activity could be decreased, which causes subsequent increases in ADMA levels. In a study conducted by Coccaro et al. [30] 2016, it was reported that impulsive aggression and the presence of personality disorder were both related to elevated oxidative stress, which was proven with increased blood levels in terms of 8-hydroxy-2'-deoxyguanosine. Moreover, inhibition of antioxidant pathways was reported. These changes are partially related to impulsivity scores in patients with BPD [1].
In this study, it was discovered that patients with SMB had lower vitamin E levels compared to the control group. It is known that vitamin E can have role as an antioxidant in the prevention of lipid peroxidation formation [31]. It has also been reported that vitamin E has a positive effect on oxidative and inflammatory conditions and alleviates depressive symptoms [26]. The decreased vitamin E levels can also be interpreted as a compensatory process for decreasing oxidative stress. In a normal physiological state, ROS are detoxified by the antioxidant enzymes. However, excessive production of ROS can cause DNA and lipid damage, leading to apoptosis and cell membrane damage. As a result, mood-regulating mechanisms may be disrupted [32]. Studies have shown that the use of antioxidant compounds in mental disorders heal the symptoms of the disease [25].
Despite the limited number of studies on oxidative stress and personality disorders, the available findings can be interpreted in the context of a larger body of research that connects psychological states and biological processes [5]. In our study, the results were compatible with the findings of studies that investigated other mental disorders, such as schizophrenia, where significant changes were observed in oxidative/nitrosative parameters [33].
To the best of our knowledge, this is the first report that identifies a relationship between oxidative stress by MDA and ADMA in BPD. In light of these findings, it was thought that BPD, which is characterized by SMB, may be associated with increased oxidative stress levels. This study has certain limitations. The number of patients with BPD included in the study was small and the inclusion of only female participants resulted in a limitation in terms of generalization of the findings for both sexes.
In conclusion, due to the biochemical nature of the BPD syndrome, patients with BPD are associated with dysregulation of internal and external stress that causes SMB as well as various impulsive behaviors to reduce psychological pain. In the present study, it was determined that ADMA and MDA levels, which reflect oxidative stress, were higher while vitamin E levels, which act as antioxidants, were determined to be lower in BPD patients with SMB. Furthermore, depression and anxiety scores were correlated with ADMA and MDA. Studies on the effects of oxidative stress on BPD are still rarely done. Future studies should be conducted with larger samples that include both sexes while investigating the relationship between oxidant/antioxidant imbalances and the clinical characteristics of patients with BPD.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Selda Telo. Data curation: Selda Telo, Sema Baykara. Formal analysis: Selda Telo, Sema Baykara. Methodology: Selda Telo, Sema Baykara. Supervision: Selda Telo. Writingâoriginal draft: Selda Telo, Sema Baykara. Writingâreview & editing: Selda Telo, Sema Baykara.
Funding Statement
None
Figure 1.
Comparison of ADMA and MDA levels between groups. ADMA, asymmetric dimethyl-arginine; MDA, malondialdehyde; BPD, borderline personality disorder; NSMB, non-self-mutilating behavior; SMB, self-mutilating behaviors.
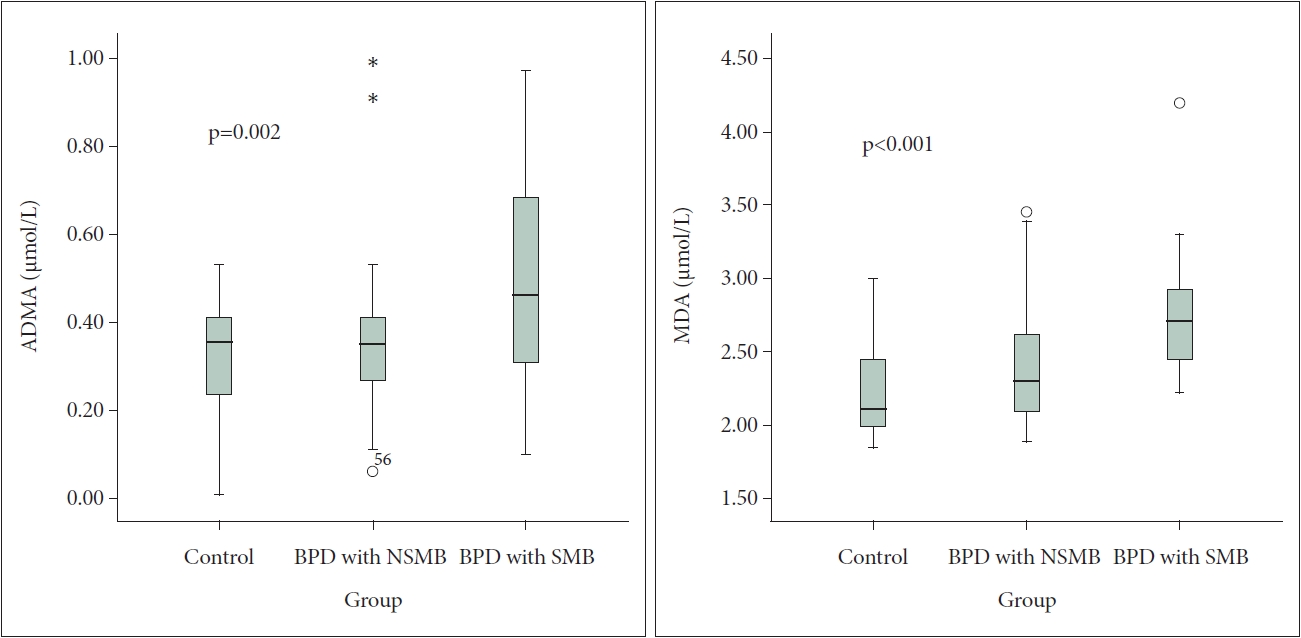
Table 1.
Demographic datas and laboratory results and BAI and BDI scores of the groups
| Parameters | BPD with SMB (N=30) | BPD with NSMB (N=30) | Control (N=30) | p |
|---|---|---|---|---|
| Age (yr) | 27.80±8.37 | 30.06±9.13 | 26.20±5.77 | 0.160 |
| Vitamin A (mg/L) | 0.46±0.08 | 0.47±0.08 | 0.49±0.13 | 0.350 |
| Vitamin E (mg/L) | 8.32±3.23* | 8.64±2.54 | 9.91±3.10 | 0.040 |
| BAI | 29.80±15.80â | 19.06±11.02⥠| 5.68±4.99 | <0.001 |
| BDI | 27.33±13.93â | 23.66±9.14â | 5.10±3.12 | <0.001 |
Table 2.
ADMA and MDA levels of groups
| Parameters | BPD with SMB (N=30) | BPD with NSMB (N=30) | Control (N=30) | p |
|---|---|---|---|---|
| ADMA (”mol/L) | 0.49±0.25* | 0.35±0.15â | 0.30±0.14 | 0.002 |
| MDA (”mol/L) | 2.74±0.40⥠| 2.41±0.41§ | 2.25±0.33 | 0.000 |
REFERENCES
1. MacDowella KS, MarsĂĄd MD, Buenachea E, Villatoro JML, Moreno B, et al. Inflammatory and antioxidant pathway dysfunction in borderline personality disorder. Psychiatry Res 2020;284:112782


2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed). Arlington, VA: American Psychiatric Publishing; 2013.
3. Zimmerman M, Mattia JI. Axis I diagnostic comorbidity and borderline personality disorder. Compr Psychiatry 1999;40:245-252.


4. Anderson G. Pathoetiology and pathophysiology of borderline personality: role of prenatal factors, gut microbiome, mu- and kappa-opioid receptors in amygdala-PFC interactions. Prog Neuropsychopharmacol Biol Psychiatry 2020;98:109782


5. Lee RJ, Gozal D, Coccaro EF, Fanning J. Narcissistic and borderline personality disorders: relationship with oxidative stress. J Pers Disord 2020;34:6-24.


6. Mopuru R, Menon V. Cycloxoygenase-2 inhibitors: a novel treatment option for borderline personality disorder? Indian J Psychol Med 2023 Feb 14 [Epub]. https://doi.org/10.1177/02537176231155733.

7. Saccora LF, Schilliger Z, Dayer A, Perroud N, Piguet C. Inflammation, anxiety, and stress in bipolar disorder and borderline personality disorder: a narrative review. Neurosci Biobehav Rev 2021;127:184-192.


8. Sher L, Rutter SB, New AS, Siever LJ, Hazlett EA. Gender differences and similarities in aggression, suicidal behaviour, and psychiatric comorbidity in borderline personality disorder. Acta Psychiatr Scand 2019;139:145-153.



9. Minutolo G, Petrali A, Dipasquale S, Aguglia E. Nitric oxide in patients with schizophrenia: the relationship with the severity of illness and the antipsychotic treatment. Expert Opin Pharmacother 2012;13:1989-1997.


10. Ustundag MF, Ozcan H, Gencer AG, Yilmaz ED, UÄur K, Oral E, et al. Nitric oxide, asymmetric dimethylarginine, symmetric dimethylarginine and L-arginine levels in psychotic exacerbation of schizophrenia and bipolar disorder manic episode. Saudi Med J 2020;41:38-45.



11. Canpolat S, Kırpınar I, Deveci E, Aksoy H, Bayraktutan Z, Eren I, et al. Relationship of asymmetrical dimethylarginine, nitric oxide, and sustained attention during attack in patients with major depressive disorder. Sci World J 2014;2014:624395




12. Aydemir Ă, ĂubukçuoÄlu Z, Erdin S, TaĆ C, Onur E, Berk M. Oxidative stress markers, cognitive functions, and psychosocial functioning in bipolar disorder: an empirical cross-sectional study. Braz J Psychiatry 2014;36:293-297.


13. Sorias S, Saygılı R, Elbi H. [DSM-III-R structured clinical interview Turkish version SCID-II personality disorders form]. Bornova, Izmir: Ege University Press; 1990,Turkish.
14. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988;56:893-897.


15. Hisli N. [A Study on the Validity of the Beck Depression Inventory]. Turk Psikol Derg 1988;22:118-126. Turkish.
16. Jorgensen A, Knorr U, Soendergaard MG, Lykkesfeldt J, Fink-Jensen A, Poulsen HE, et al. Asymmetric dimethylarginine in somatically healthy schizophrenia patients treated with atypical antipsychotics: a case-control study. BMC Psychiatry 2015;15:67




17. Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 2001;357:593-615.




18. Rujescu D, Giegling I, Mandelli L, Schneider B, Hartmann AM, Schnabel A, et al. NOS-I and -III gene variants are differentially associated with facets of suicidal behavior and aggression-related traits. Am J Med Genet B Neuropsychiatr Genet 2008;147B:42-48.


19. Zhoua QG, Zhua XH, Nemes AD, Zhua DY. Neuronal nitric oxide synthase and affective disorders. IBRO Rep 2018;5:116-132.



20. Köse SS, ErbaĆ O. Personality disorders diagnosis, causes, and treatments. Demiroglu Sci Univ Florence Nightingale J Transplant 2020;5:22-31.

21. Reif A, Jacob CP, Rujescu D, Herterich S, Lang S, Gutknecht L, et al. Influence of functional variant of neuronal nitric oxide synthase on impulsive behaviors in humans. Arch Gen Psychiatry 2009;66:41-50.


22. Reif A, Kiive E, Kurrikoff T, Paaver M, Herterich S, Konstabel K, et al. A functional NOS1 promoter polymorphism interacts with adverse environment on functional and dysfunctional impulsivity. Psychopharmacology (Berl) 2011;214:239-248.



23. Eli R, Fasciano J. A chronopharmacological diagnostic test and treatment for bipolar disorder and depression: nitric oxide release during sleep causes it to become depressogenic in a subset of patients. Med Hypotheses 2006;66:72-75.


24. Kielstein H, Suntharalingam M, Perthel R, Song R, Schneider SM, Martens-Lobenhoffer J, et al. Role of the endogenous nitric oxide inhibitor asymmetric dimethylarginine (ADMA) and brain-derived neurotrophic factor (BDNF) in depression and behavioural changes: clinical and preclinical data in chronic kidney disease. Nephrol Dial Transplant 2015;30:1699-1705.


25. Forte ARCC, Lessa PHC, Chaves Filho AJM, Aquino PEA, Brito LM, Pinheiro LC, et al. Oxidative stress and inflammatory process in borderline personality disorder (BPD): a narrative review. Braz J Med Biol Res 2023;56:e12484



26. Manosso LM, Camargo A, Dafre AL, Rodrigues ALS. Vitamin E for the management of major depressive disorder: possible role of the anti-inflammatory and antioxidant systems. Nutr Neurosci 2020;25:1310-1324.


27. WĂłjcicka G, Jamroz-WiĆniewska A, Czechowska G, Korolczuk A, Marciniak S, BeĆtowsk J. The paraoxonase 1 (PON1), platelet-activating factor acetylohydrolase (PAF-AH) and dimethylarginine dimethylaminohydrolase (DDAH) activity in the metformin treated normal and diabetic rats. Eur J Pharmacol 2016;789:187-194.


28. HurĆitoÄlu O, Â Orhan FĂ, Â KurutaĆ EB, DoÄaner A, DurmuĆ HT, Kopar H. Diagnostic performance of increased malondialdehyde level and oxidative stress in patients with schizophrenia. Noro Psikiyatr Ars 2021;58:184-188.


30. Coccaro EF, Lee R, Gozal D. Elevated plasma oxidative stress markers in individuals with intermittent explosive disorder and correlation with aggression in humans. Biol Psychiatry 2016;79:127-135.


31. DâSouza B, DâSouza V. Oxidative injury and antıoxidant vitamins E and C in schizophrenia. Indian J Clin Biochem 2003;18:87-90.




32. Kamal NAM, Loo JL, Goon JA, Damanhuri HA, Sharip S, Saini SM, et al. Oxidative stress biomarkers in bipolar disorder with suicidal behavior: a systematic review. J Pharm Negat Results 2019;10:6-15.

33. Zincir SB, Zincir S. [Role of Asymmetric Dimethylarginine in Psychiatric Disorders]. Curr Approaches Psychiatry 2014;6:355-362. Turkish.







