|
 |
- Search
| Psychiatry Investig > Volume 12(3); 2015 > Article |
Abstract
Objective
We hypothesize that the amplitude of low-frequency fluctuations (ALFF) is involved in the altered regional baseline brain function in social anxiety disorder (SAD). The aim of the study was to analyze the altered baseline brain activity in drug-naive adult patients with SAD.
Methods
We investigated spontaneous and baseline brain activities by obtaining the resting-state functional magnetic resonance imaging data of 20 drug-naïve adult SAD patients and 19 healthy controls. Voxels were used to analyze the ALFF values using one- and two-sample t-tests. A post-hoc correlation of clinical symptoms was also performed.
Results
Our findings show decreased ALFF in the bilateral insula, left medial superior frontal gyrus, left precuneus, left middle temporal gyrus, right middle temporal pole, and left fusiform gyrus of the SAD group. The SAD patients exhibited significantly increased ALFF in the right inferior temporal gyrus, right middle temporal gyrus, bilateral middle occipital gyrus, orbital superior frontal gyrus, right fusiform gyrus, right medial superior frontal gyrus, and left parahippocampal gyrus. Moreover, the Liebowitz Social Anxiety Scale results for the SAD patients were positively correlated with the mean Z values of the right middle occipital and right inferior occipital but showed a negative correlation with the mean Z values of the right superior temporal gyrus and right medial superior frontal gyrus.
Social anxiety disorder (SAD), also known as social phobia, is the persistent fear of social or performance situations in which the person is exposed to unfamiliar people or to possible public scrutiny.1 Resting-state functional magnetic resonance imaging (fMRI) is a novel technique that has several potential advantages over task performance or stimulation in terms of clinical applicability.234 Previous studies have found altered brain functions in the medial prefrontal cortex (MPFC) and limbic regions, including the amygdala, hippocampus, and insula, of SAD patients.5678910111213 However, investigation on SAD at the baseline levels or studies on the spontaneous activity of the brain must be conducted to obtain a clearer understanding of the neurobiology of the disorder.314 Furthermore, a relationship has been observed between the altered spontaneous activity of the functionally relevant cortices and clinical features.14 Thus, studying the spontaneous cerebral activity in drug-naïve adult patients with SAD may provide vital information for characterizing the neuropathophysiological mechanisms underlying the disorder.
The amplitude of low-frequency fluctuations (ALFF), which is a widely accepted resting-state data analysis tool that can provide information on regional spontaneous neuronal activity (SNA), has been extensively used to study mental disorders.15 Brain diseases may exhibit abnormal local SNA and/or inter-regional SNA synchronization while ALFF reflects the extent of SNA. Hence, the changed SNA of these regions by ALFF may be implicated in the underlying pathophysiology in disease.15 Thus, this method has been suggest to investigate the functional modulations and characterize the neuropsychological changes in the resting state in patients with various clinical populations.161718 Although several studies have demonstrated the altered resting-state functional connectivity in various brain networks during SAD, to the best of our knowledge, no resting-state fMRI study has been conducted on the ALFF in SAD. By using resting-state fMRI and ALFF analysis, the current research focused on the local coherence of spontaneous activity of drug-naïve patients with SAD. This study aims to determine whether 1) the ALFF in some brain areas of SAD patients are aberrant, and 2) these changes are related to the measured clinical severity of the disorder.
Twenty patients (22.90┬▒2.99 years old, all right-handed) were consecutively recruited from West China Hospital of Sichuan University. A diagnosis of SAD was determined by consensus between two attending psychiatrists using the Structured Clinical Interview for DSM-IV (SCID) Patient Edition.19 The exclusion criteria included history of neurological and psychiatric disease and diagnosis of other mental disorders, except SAD; existence of an organic brain disorder; alcohol or drug abuse; and pregnancy or any physical illness such as hepatitis, brain tumor, or epilepsy, as assessed from the medical records. No gross abnormalities were observed in the brain MRI scans (i.e., T1-weighted and T2-weighted images) of any of the subjects when examined by an experienced neuroradiologist. The SAD patients have yet to receive psychotherapy and psychiatric medications.
Twenty age- and education-matched healthy controls (HCs) (mean age=21.38┬▒3.77 years, all right-handed) were recruited from the local community via poster advertisement and then screened using the SCID-I/P version to confirm the absence of any psychiatric or neurological illness. The HC subjects were told beforehand to cooperate in the radiological check for fear of intentional withdrawal because of some mental disorders. All HC subjects were interviewed to confirm that no history of psychiatric illness exists among their first-degree relatives. However, one person was involuntarily discontinued during MR scanning because of hypertension. Finally, image data were acquired from 19 HCs.
The participants of the two groups were evaluated using the Liebowitz Social Anxiety Scale (LSAS), State-Trait Anxiety Inventory (STAI), the Hamilton Anxiety Rating Scale (HAMA), and Hamilton Depression Rating Scale (HAMD). According to previous studies, LSAS does not replace a clinical interview for the diagnosis of social anxiety even when the score exceeds 38, which is indicative a probable SAD diagnosis. The present study was approved by the local Ethics Committee. A written informed consent was obtained from all subjects after they were provided with a complete description of the study.
Experiments were performed using a 3.0-T GE-Sigma MRI scanner (EXCITE, General Electric, Milwaukee, WI, USA). A foam padding was used to minimize head motion. Functional images were acquired using a single-shot, gradient-recalled echo-planar imaging sequence (TR=2000 ms, TE=30 ms, and flip angle=90┬░). Thirty transverse slices (FOV=24 cm, in-plane matrix=64├Ś64, slice thickness=5 mm, without gap) aligned along the anterior commisure-posterior commisure line were acquired. A total of 205 volumes were acquired for each subject, and the first 5 volumes were discarded to ensure steady-state longitudinal magnetization. Subsequently, for spatial normalization and localization, a set of high-resolution T1-weighted anatomical images in the axial orientation were acquired for each subject using a 3D spoiled-gradient recalled sequence (TR=8.5 ms, TE=3.4 ms, flip angle=12┬░, matrix size=512├Ś512├Ś156, and voxel size=0.47├Ś0.47├Ś1 mm3).
Data preprocessing was partly conducted using the Statistical Parametric Mapping software (SPM2, http://www.fil.ion.ucl.ac.uk/spm). The 200 volumes were first corrected for temporal difference in the acquisition of the different slices. Afterward, the images were realigned to the first volume for head-motion correction. For one subject, the translational or rotational parameters in these datasets exceeded ┬▒1.5 mm or ┬▒1.5┬░; these datasets were thus excluded from the analysis. We also evaluated the group differences in translation and rotation of head motion according to the following formula: where L is the length of the time series (L=200 in this study), xi, yi and zi are translations/rotations at the ith time point in the x, y and z directions, respectively.20 The fMRI images were realigned with the corresponding T1 volume and warped into a standard stereotaxic space at a resolution of 3├Ś3├Ś3 mm3 using the Montreal Neurological Institute echo-planar imaging template in SPM2. Afterward, the images were spatially smoothed via convolution with an isotropic Gaussian kernel (FWHM=8 mm).
The ALFF analysis was performed using the REST software (http://resting-fmri.sourceforge.net). The following procedure was applied to calculate the ALFF:15 after band pass filtering (0.01 Hz to 0.08 Hz)2122 and linear-trend removal, the time series was transformed into a frequency domain using fast Fourier transform (FFT) (taper percent: 0, FFT length: shortest), and the power spectrum was obtained. Given that the power of a given frequency is proportional to the square of the amplitude of this frequency component in the original time series in the time domain, the square root of the power spectrum obtained by FFT was calculated and then averaged across 0.01 Hz to 0.08 Hz at each voxel. This averaged square root was taken as the ALFF. For normalization purposes, the ALFF of each voxel was divided by the mean ALFF value within a brain mask.
A one-sample, one-sided t-test was performed within each group to determine whether the ALFF deviated from the value of 1. A two-sample t-test was performed to verify the ALFF difference between the two groups. Voxels (p<0.001, uncorrected; cluster size >10 voxels) were used to show the significant difference between the two groups. Two-sample t-tests were also performed to assess the differences in the head motions of the two groups. A post-hoc Pearson correlation analysis was also performed to investigate the relationship between the ALFF values and clinical severity. Given that these analyses were exploratory in nature, a statistical significance level of p<0.05 (uncorrected) was used.
The group demographic characteristics and neuropsychological scores are shown in Table 1. No significant difference was found in the gender, age, and education of the different groups. The LSAS total score (t=11.02, p<0.001), fear factor (t=10.90, p<0.001), and avoidance factor (t=7.88, p<0.001) of the SAD patients were significantly higher than those of the healthy controls (HCs). Significant differences were also found between HAMD (t=4.36, p<0.001) and HAMA (t=4.65, p<0.001) of the two groups. Compared with the HCs, the SAD patients showed significantly higher levels of anxiety, as assessed by STAI-T (t=8.07, p<0.001) and prescanning STAI-S (t=4.73, p<0.001). No difference was found in the post-scanning STAI-S (t=1.84, p=0.073) of the patients and the HCs. Moreover, no significant head motion difference was observed between the two groups (shift: t=-1.15, p=0.259; rotation: t=-0.95, p=0.351) (Table 1).
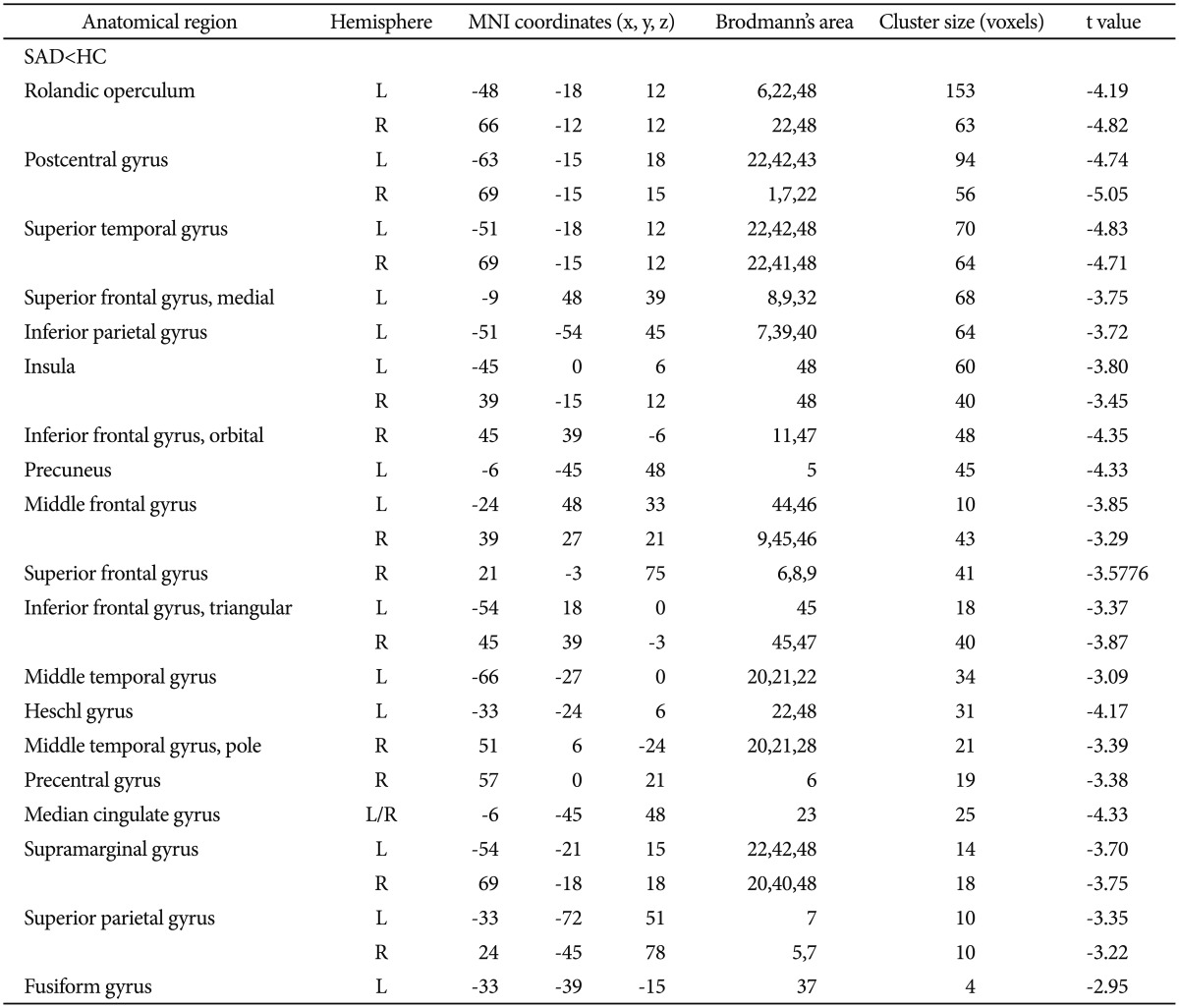
The one-sample t-tests of the ALFF analysis showed significantly higher ALFF in the posterior cingulate cortex/precuneus (PCC/Pcun), MPFC, and bilateral angular gyrus of the HCs and SAD patients (p<0.001, uncorrected) (Figure 1).
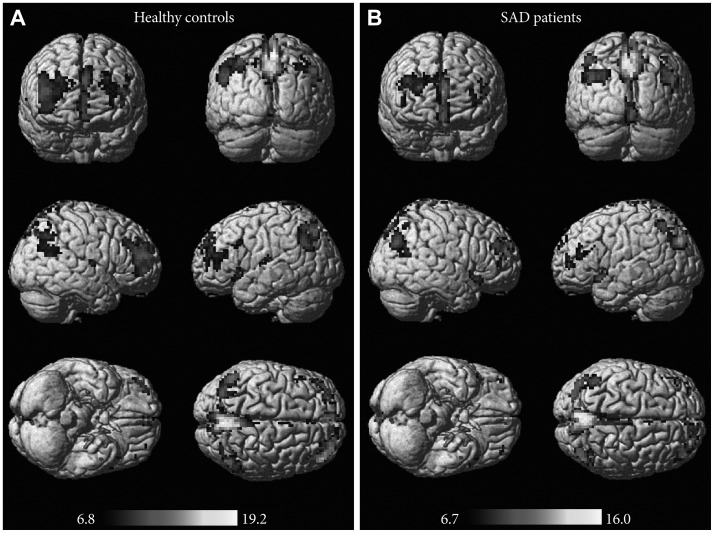
The two-sample t-test showed significant difference in some brain areas of the two groups (p<0.001, uncorrected) (Figure 2). In the SAD group, the brain regions with decreased ALFF included the bilateral rolandic operculum, postcentral gyrus, superior temporal gyrus, middle frontal gyrus, triangular inferior frontal gyrus, supramarginal gyrus, superior parietal gyrus, and insula (Table 2, Figure 2). Conversely, the SAD patients exhibited significantly increased ALFF in these cortices, including the right inferior temporal gyrus and right middle temporal gyrus (these two areas were merged into one cluster), as well as in the bilateral middle occipital gyrus, orbital superior frontal gyrus, right inferior occipital gyrus, right orbital medial frontal gyrus, and other regions (Table 3, Figure 2).
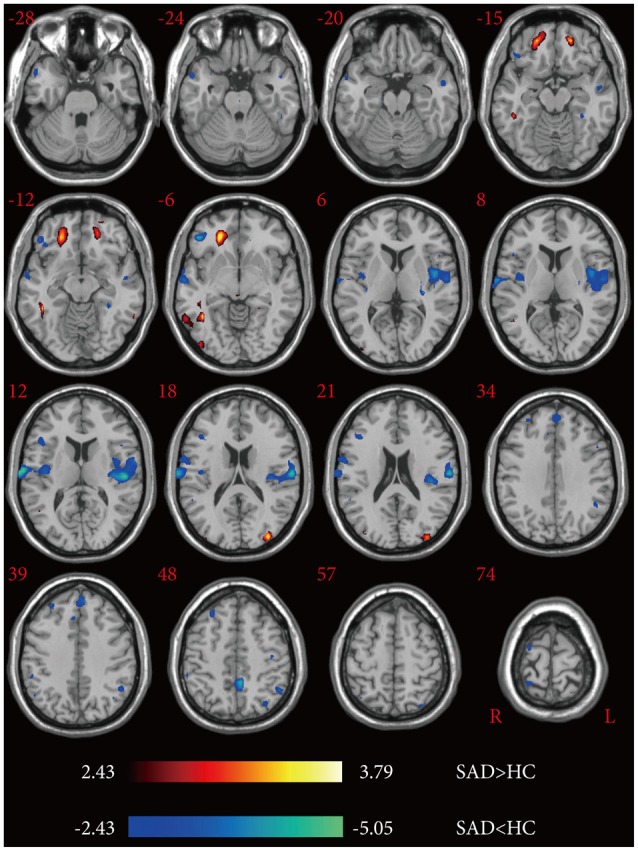
The LSAS values for the SAD patients showed a significantly positive correlation with the mean Z values of the right middle occipital and right inferioral occipital but was negatively correlated with those of the right superior temporal gyrus and right medial superior frontal gyrus (p<0.05) (Figure 3). In addition, the other brain regions with aberrant ALFF showed no significant correlation with the LSAS values.
To the best of our knowledge, the present study is the first to report on an association between ALFF at rest and the severity of social anxiety symptoms in patients, particularly drug-naive adults. We found a significant difference in the ALFF of the SAD patients and the HCs. The SAD group showed altered ALFF in insula, medial superior frontal gyrus, PCC/Pcun, temporal lobe and fusiform gyrus, and occipital lobe. In addition, the LSAS values of SAD patients showed a positive correlation with the mean Z values of the right middle occipital and right inferioral occipital but negatively with that of the right superior temporal gyrus and right medial superior frontal gyrus.
Both STAI-T and prescanning STAI-S results showed that the SAD patients had higher anxiety levels than HCs, whereas post-scanning STAI-S showed no significant differences between the anxiety levels of the two groups. This finding is consistent with those of previous studies.23 SAD patients often abnormally feel anxious and worried about embarrassing themselves in unfamiliar situations.24 This mental state is different from scan-related anxiety.25 The clinical scale results suggest that the symptoms of SAD patients change with the social situation; that is, these patients do not develop anxiety symptoms until they find themselves in a social setting. The decrease in the post-scanning anxiety level indicates that anxiety anticipation reached remission and was released when the subjects were removed from the social setting. Thus, anxiety anticipation is vital to the pathopsychological mechanism of SAD. However, this finding requires further verification by a combined study of tasking-state fMRI and behavioral tests.
Our results show decreased ALFF in the insula of SAD patients. By contrast, a number of studies reported that hyperfunction5891126 occurs more frequently than hypofunction10 in insula.27 In accordance with previous studies, we hypothesize that the persistent hyperactivity in the insula of anxious patients disrupts the spontaneous neural activity and function for anticipatory processing in the insula.1427 These differences in insula activity, which range from tasking to the resting state, may be explained by the implicit sequence learning during the resting state; this type of learning is a cognitive task that is not aimed at symptom provocation.10 In addition, this area may be critical for the extraction and selection of task-relevant information and has been implicated in inhibitory control.28 Thus, the insula showed hyperfunction during symptom provocation. Moreover, the capacity of this brain region to deal with a cognitive task even in the resting state was disrupted.
The one-sample t-tests in the ALFF analysis showed significantly higher ALFF in the PCC/Pcun, MPFC, and bilateral AG of the HCs (Figure 1A). This pattern is consistent with that of the DMN.2 In social cognition and "theory of mind", the PCC/Pcun is sometimes referred to as a pivotal hub of the DMN.2930 Moreover, the PCC/Pcun is more active when socially relevant emotional stimuli are perceived31 and is involved in a network of self-consciousness and self-related mental representations.32 Social cognition and "theory of mind", which are two preeminent cognitive behavioral models of social anxiety,3334 have described that self-focused attention in SAD and misinterpretation of interoceptive information increase access to negative thoughts and feelings; these negative reactions prevent individuals from gaining accurate information (about the situation and the responses of others) that could diffuse the negative expectations.35 Thus, our study provided further evidence that in SAD, Pcun dysfunction results in inaccurate evaluation of the self-emotional state as well as in incomplete processing of self-related representations. Given the role of PCC/Pcun in self-state perception and attribution, our findings support the hypothesis that PCC/Pcun can suspend its function within the DMN.36 We propose that the impairment of PCC/Pcun in the DMN of SAD patients may be relevant in the development of the feeling of wariness of others' judgment as well as in the remaining high-level, self-focused attention.
The aberrant ALFF of SAD patients are represented in the right medial superior frontal gyrus. In previous studies, the activities in the MPFC of SAD subjects either increased or decreased but showed no significant difference during task performance.3738 The MPFC activity possibly reflects an interaction between cognitive processing and emotional state.2639 Meanwhile, the prefrontal cortex activation appears to correlate with the self-referential magnitude and higher evaluation processes,263039 particularly with the construction of self-relevant mental simulation in the form of memories.36 Our findings, along with the hypotheses on MPFC, suggest that MPFC is significantly involved in the prominent psychopathological mechanism of SAD. Interestingly, the orbitofrontal/MPFC has been hypothesized to have a role in the inhibition or extinction of excessive corticolimbic activity in anxiety disorders,4041 However, our results show no evidence of excessive amygdala-hippocampal activity in the patients during the resting state. The more likely frontal compensation, does not prevent limbic overactivity, as shown by the aberrant ALFF in the resting-state frontal cortex. Whether this result indicates a compensatory mechanism in the brain requires further research. Meanwhile, the prefrontal cortex may be involved in the internal representation of the social environment42 and may also have a role in our ability to read the mental states of other people.43 The frontal dysfunction from this study possibly reflect the dysfunction of the cortical regions that directly contribute to the etiology of SAD. Our results on the bilateral medial superior frontal gyrus have not been previously reported. These results are associated with the relationship between the SAD subtype and other factors and thus require further investigation.
In this study, decreased ALFF was observed in the temporal cortex, including the bilateral superior temporal gyrus, superior parietal gyrus, left inferior parietal gyrus, left middle temporal gyrus, and right middle temporal pole. These results are inconsistent with the significantly increased ALFF in the right inferior/middle temporal gyrus and right fusiform gyrus (Figure 2, Table 2) but partly agree with the results of previous tasking-state studies on the fusiform of SAD subjects.81135 The fusiform can form a higher-sensory cortex, which is involved in the perceptual representation of social stimuli.34 Alternatively, the increased ALFF in the fusiform gyrus may be due to the higher vigilance and alertness of SAD patients, whereas the decreased ALFF in the fusiform gyrus may be due to avoidance. This anterior interpretation is supported by the relationships between the mean Z values of ALFF in the right superior temporal gyrus, whereas the LSAS results were mostly correlated with the fear factor.35
The negative correlation between the mean Z values of ALFF in the right superior temporal gyrus and the LSAS scores further provides evidence for the functional impairments of SAD patients in terms of resting-state facial perception and social evaluation. Although the function of the temporal poles is not well understood, damaged temporal poles can impair the ability to use the knowledge we gain from the world through our experiences; this knowledge is important for our ability to mentalize.44 Thus, the abnormal ALFF values in the aforementioned brain regions indicate a functional defect in the SAD patients to evaluate the current situation correctly using their experience and/or by observing the behavior of others. The functional imaging results suggest that temporal cortex dysfunction has a vital function in the psychopathology of SAD.
In this study, the significantly reduced ALFF in the cortices, including the pre- and post-central gyrus, and increased ALFF in the middle and inferior occipital gyruses contribute to perceptual impairments of the sensorimotor cortex (SMC) and supersensitivity of the visual cortex of SAD patients. Some of these results are consistent with the findings of previous tasking-state fMRI studies and suggest that sensorimotor and the visual cortex showed significantly stronger BOLD responses to social threat in SAD patients.7 Moreover, the significant positive correlation between the LSAS scores and the mean ALFF Z values in the right middle occipital gyrus and right inferioral occipital gyrus of the patients further provide evidence for the resting-state hyperactivity of the visual cortex in SAD. Along with our current data, these functional imaging results suggest a dysfunction in the SMC area and visual cortex of SAD patients. Thus, the hypothesis that SAD patients are over-aroused and exhibit hyperprosexia for social information45 as a result of hyperactivity in the visual cortex was confirmed.
Several limitations must be considered in this research. First, the amygdala has a vital function in fear. However, in this study, we did not find significant differences in the ALFF of the amygdalae of the two groups, possibly because of the relatively low fMRI signal-to-noise ratio in the basal ganglia in resting-state fMRI. Second, head motion is relatively a greater issue for anxiety-disorder patients than for the controls during fMRI. In this study, we did not find significant differences in the head motion of the two groups. However, a more restricted head motion should yield more accurate and more reliable results. Third, the physiological noise induced by cardiac and respiratory rhythms is a significant factor in functional neuroimaging studies, particularly in persons with anxiety disorders.15 In this study, we were unable to resolve the bias generated by these noises. Future studies could focus on a few specific regions of interest and utilize multiple research methods, such as resting- and tasking-state fMRI and PET. In addition, a larger group of subjects would increase the statistical power of the study.
Our findings indicate differential impairments or dysfunctions in the neural activity of the insula, PCC/Pcun, and frontal and temporal cortices in SAD patients. These results suggest that the regions with abnormal spontaneous activities are involved in the underlying pathophysiology of SAD patients. These findings also support the results of a tasking-state study, which showed that abnormal responses may be partly due to abnormal spontaneous baseline neural activity.
Acknowledgments
This research was partly supported by the 863 Program (Grant No. 2008AA02Z408), the National Natural Science Foundation (Grant No. 30900361), the 973 Project (Grant No. 2008CB517407), the Distinguished Young Scholars of Sichuan (Grant No. 2011JQ0005), and the Programs for New Century Excellent Talents in University (Grant No. NCET-10-0596).
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision. Washington DC: American Psychiatric Association; 2000.
2. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A 2001;98:676-682. PMID: 11209064.



3. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007;8:700-711. PMID: 17704812.


4. Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage 2007;37:1083-1090. PMID: 17719799.


5. Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, L├źngstr├Čm B, et al. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am J Psychiatry 2001;158:1220-1226. PMID: 11481154.


6. Furmark T, Tillfors M, Marteinsdottir I, Fischer H, Pissiota A, L├źngstr├Čm B, et al. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Arch Gen Psychiatry 2002;59:425-433. PMID: 11982446.


7. Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry 2002;59:1027-1034. PMID: 12418936.


8. Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WH. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biol Psychiatry 2004;56:921-930. PMID: 15601601.


9. Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007;164:1476-1488. PMID: 17898336.



10. Sareen J, Campbell DW, Leslie WD, Malisza KL, Stein MB, Paulus MP, et al. Striatal function in generalized social phobia: a functional magnetic resonance imaging study. Bio Psychiatry 2007;61:396-404. PMID: 17097072.

11. Gentili C, Gobbini MI, Ricciardi E, Vanello N, Pietrini P, Haxby JV, et al. Differential modulation of neural activity throughout the distributed neural system for face perception in patients with Social Phobia and healthy subjects. Brain Res Bull 2008;77:286-292. PMID: 18771714.


12. Damsa C, Kosel M, Moussally J. Current status of brain imaging in anxiety disorders. Curr Opin Psychiatry 2009;22:96-110. PMID: 19122541.


13. Engel K, Bandelow B, Gruber O, Wedekind D. Neuroimaging in anxiety disorders. J Neural Transm 2009;116:703-716. PMID: 18568288.


14. Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry 2009;66:1361-1372. PMID: 19996041.


15. Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 2007;2:83-91. PMID: 16919409.
16. Zhang Z, Lu G, Zhong Y, Tan Q, Chen H, Liao W, et al. fMRI study of mesial temporal lobe epilepsy using amplitude of low-frequency fluctuation analysis. Hum Brain Mapp 2010;31:1851-1861. PMID: 20225278.



17. Ji GJ, Zhang Z, Zhang H, Wang J, Liu DQ, Zang YF, et al. Disrupted causal connectivity in mesial temporal lobe epilepsy. PLoS One 2013;8:e63183PMID: 23696798.



18. Chen S, Wu X, Lui S, Wu Q, Yao Z, Li Q, et al. Resting-state fMRI study of treatment-naive temporal lobe epilepsy patients with depressive symptoms. Neuroimage 2012;60:299-304. PMID: 22178816.


19. First MB, Gibbon M. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). In: Hilsenroth MJ, Segal DL, editor. Comprehensive Handbook of Psychological Assessment, Vol. 2: Personality Assessment. New Jersey: John Wiley & Sons Inc, 2004, p. 134-143.
20. Liao W, Chen H, Feng Y, Mantini D, Gentili C, Pan Z, et al. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage 2010;52:1549-1558. PMID: 20470894.


21. Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage 1998;7:119-132. PMID: 9558644.


22. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995;34:537-541. PMID: 8524021.


23. Taylor CT, Bomyea J, Amir N. Attentional bias away from positive social information mediates the link between social anxiety and anxiety vulnerability to a social stressor. J Anxiety Disord 2010;24:403-408. PMID: 20207102.



24. Clark DM, McManus F. Information processing in social phobia. Biol Psychiatry 2002;51:92-100. PMID: 11801234.


25. Grey SJ, Price G, Mathews A. Reduction of anxiety during MR imaging: a controlled trial. Magn Reson Imaging 2000;18:351-355. PMID: 10745145.


26. Phan KL, Taylor SF, Welsh RC, Decker LR, Noll DC, Nichols TE, et al. Activation of the medial prefrontal cortex and extended amygdala by individual ratings of emotional arousal: a fMRI study. Bio Psychiatry 2003;53:211-215. PMID: 12559653.

27. Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry 2007;164:318-327. PMID: 17267796.


28. Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A 1999;96:8301-8306. PMID: 10393989.



29. Brune M, Brune-Cohrs U. Theory of mind--evolution, ontogeny, brain mechanisms and psychopathology. Neurosci Biobehav Rev 2006;30:437-455. PMID: 16239031.


30. Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci 2006;7:268-277. PMID: 16552413.


31. Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Rauch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress Anxiety 2008;25:496-505. PMID: 17595018.


32. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 2006;129:564-583. PMID: 16399806.


33. Clark DM, Wells A. A Cognitive Model of Social Phobia. In: Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, editor. Social Phobia: Diagnosis, Assessment, and Treatment. New York: Guilford Press, 1995, p. 69-93.
34. Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behav Res Ther 1997;35:741-756. PMID: 9256517.


35. Warwick JM, Carey P, Jordaan GP, Dupont P, Stein DJ. Resting brain perfusion in social anxiety disorder: a voxel-wise whole brain comparison with healthy control subjects. Prog Neuropsychopharmacol Bio Psychiatry 2008;32:1251-1256. PMID: 18485554.

36. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 2008;1124:1-38. PMID: 18400922.


37. Zhao XH, Wang PJ, Li CB, Hu ZH, Xi Q, Wu WY, et al. Altered default mode network activity in patient with anxiety disorders: an fMRI study. Eur J Radiol 2007;63:373-378. PMID: 17400412.


38. Br├╝hl AB, Rufer M, Delsignore A, Kaffenberger T, J├żncke L, Herwig U. Neural correlates of altered general emotion processing in social anxiety disorder. Brain Res 2011;1378:72-83. PMID: 21215728.


39. Simpson JR Jr, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: I. During cognitive task performance. Proc Natl Acad Sci U S A 2001;98:683-687. PMID: 11209065.



40. LeDoux J. Fear and the brain: where have we been, and where are we going? Bio Psychiatry 1998;44:1229-1238. PMID: 9861466.

41. Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Bio Psychiatry 2002;51:68-80. PMID: 11801232.

42. Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci 2003;4:165-178. PMID: 12612630.


43. Frith CD. The social brain? Philos Trans R Soc Lond B Biol Sci 2007;362:671-678. PMID: 17255010.



44. Funnell E. Evidence for scripts in semantic dementia: implications for theories of semantic memory. Cogn Neuropsychol 2001;18:323-341. PMID: 20945219.


45. Schultz LT, Heimberg RG. Attentional focus in social anxiety disorder: potential for interactive processes. Clin Psychol Rev 2008;28:1206-1221. PMID: 18555570.


Figure┬Ā1
Results of the one-sample t-test within a group. The one-sample t-tests for the ALFF analysis show significantly higher ALFF in the posterior cingu-late cortex/precuneus, medial prefrontal cortex, and bilateral angular gyrus of (A) healthy controls and (B) SAD patients (p<0.01, uncorrected). ALFF: amplitude of low-frequency fluctuations, SAD: social anxiety disorder.
Figure┬Ā2
Results of the two-sample t-test within groups. A two-sample t-test showed significant difference in some brain areas of the two groups (p<0.001, uncorrected). The blue clusters indicate decreased ALFF brain regions in the SAD group (t values: upper color bar), whereas the red clusters represent increased ALFF brain regions (t values: bottom color bar). ALFF: amplitude of low-frequency fluctuations, SAD: social anxiety disorder, HC: healthy control.
Figure┬Ā3
Results of the correlation analysis between LSAS (LSAS-F: fear factor, LSAS-A: avoidance factor) values and ALFF in the SAD group. This scatter diagram shows the positive and negative correlations of LSAS with the mean Z values of the altered ALFF regions in SAD patients at the threshold of p<0.05 via post-hoc correlation analysis. LSAS: liebowitz social anxiety scale, ALFF: amplitude of low-frequency fluctuations, SAD: social anxiety disorder, MNI: Montreal Neurological Institute.

Table┬Ā1
Neuropsychological data and group characteristics

Data from questionnaires are presented in terms of mean score (M) and standard deviation (SD) in SAD and HC groups. The p values were obtained by two-sample two-tailed t-test. SAD: social anxiety disorder, HC: healthy control, LSAS: Liebowitz Social Anxiety Scale, HAMA: Hamilton Anxiety Rating Scale, HAMD: Hamilton Depression Rating Scale, STAI: State-Trait Anxiety Inventory, T: trait, S: state