7,8-Dihydroxyflavone, a Tropomyosin-Kinase Related Receptor B Agonist, Produces Fast-Onset Antidepressant-Like Effects in Rats Exposed to Chronic Mild Stress
Article information
Abstract
Objective
Brain-derived neurotrophic factor (BDNF) and its specific receptor, tropomyosin-related kinase (TrkB), play important roles in treating depression. In this experiment, we examined whether 7,8-dihydroxyflavone, a novel potent TrkB agonist, could reverse the behavioral and biochemical abnormalities induced by the chronic mild stress (CMS) paradigm in rats.
Methods
SD rats were exposed to a battery of stressors for 56 days. 7,8-dihydroxyflavone (5 and 20 mg/kg) were administered intraperitoneally during the last 28 days of the CMS paradigm. Rats were tested in sucrose consumption test (SCT), forced-swimming test (FST) and elevated T-maze (ETM). Serum corticosterone levels and hippocampal BDNF levels of the rats were measured.
Results
Four-week CMS on the rats induced their depression-like behavior in SCT. The CMS-reduced sucrose consumption was reversed starting from 7 days after the 7,8-dihydroxyflavone (20 mg/kg) treatment and remained across the subsequent treatment regime. 7,8-dihydroxyflavone, when given at 5 mg/kg for 3 weeks, reduced the immobility time in the FST in the CMS-subjected rats. Additionally, the 4-week treatment with 7,8-dihydroxyflavone (20 mg/kg) attenuated the CMS-induced increase in anxiety-like behavior in the ETM. For the CMS-subjected rats, 7,8-dihydroxyflavone treatment dose-dependently reduced their serum corticosterone levels but increased their hippocampal BDNF levels only at 5 mg/kg.
Conclusion
7,8-dihydroxyflavone was beneficial for both depression and anxiety-like behaviors, and may exert fast-onset antidepressant effects. This provides a new insight into the pharmacological management of depression.
INTRODUCTION
Brain-derived neurotrophic factor (BDNF), involved in the development and activity-dependent regulation of neuronal structures, is the most prominent member of the neurotrophin family.1 Accumulating evidence suggests that BDNF may be involved in the pathophysiology of major depressive disorder and that the action of antidepressants could be mediated by their action on the BDNF system.2345 Specifically, depressed patients have low levels of BDNF, which can be reversed by the treatment with antidepressants.6 BDNF exerts its effects by binding to tropomyosin-kinase related receptor B (TrkB).789 TrkB signals through mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K) and phospholipase C-γ1, and is important for the neurogenesis, synaptic function and plasticity in the adult nervous system.1011
Since BDNF has poor blood-brain barrier penetration and a short half life,12 specific small molecule TrkB agonists capable of being administered systemically and initiating TrkB activation with equal potency to BDNF, have been developed recently. 1314 Of note, one recently identified high affinity TrkB agonist, 7,8-dihydroxyflavone (DHF), has shown promising protective effects in mouse models of aging, Parkinson disease, and Alzheimer disease.14151617 Growing numbers of researchers recently sought to focus on the role of 7,8-DHF in treating depression. 1819 Chronic mild stress (CMS) paradigm is a well-known animal model of depression that causes stressed rats to exhibit depressive-like behaviors, elevated serum corticosterone levels, and decreased hippocampus BDNF levels.2021 The current study aimed to investigate whether 7,8-DHF treatment on rats can reverse the CMS induced depressive-like behaviors and biochemical alterations.
Anxiety is also a frequent and well-known consequence of chronic stress. Considerable research shows that the coexistence of depression with anxiety disorders is rather a rule than an exception,22 even though depression and anxiety are distinct psychiatric disorders. It has been demonstrated that BDNF is involved in both the pathogenesis and treatment of anxiety disorders.2324 To date, a small body of research has examined the anxiolytic effect of 7,8-DHF; for example, 7,8-DHF was reported to enhance the extinction of fear in animal models of posttraumatic stress disorder (PTSD).2526 However, a recent report failed to demonstrate the ability of 7,8-DHF to alter rats'anxiety-like behavior on an elevated plus maze.27 In the present study, we also investigated the anxiolytic effect of 7,8-DHF in CMS-subjected rats.
METHODS
Animals
Twenty-eight male Sprague-Dawley (SD) rats (BioLASCO Taiwan Co. Ltd., Taipei, Taiwan) were used. All rats were housed in groups of 3 and in the same temperature and humidity-controlled holding facility (22±2℃) on a 12 h light/dark cycle (lights on at 07:00). All animals received food and water ad libitum. The rats were allowed 1 week to acclimate to the laboratory surroundings before the beginning of the experimentation and none of them were tested until the age of 8 weeks old. All experimental procedures were evaluated and approved by the animal care committee of the National Defense Medical Center (Taiwan) with the permission code IA-CUC-04-156. All efforts were made to reduce the number of animals used and to minimize animal suffering during the experiments. All measurements were performed by individuals blind to the treatment received by the rats.
Apparatus
The elevated T-maze (ETM) was made of wood and had three arms of equal dimensions (50±12 cm2). One of the arms was enclosed by walls that were 40 cm high, and was oriented perpendicularly to two opposed open arms. The whole apparatus was elevated 50 cm above the floor. To avoid falls, a 1 cm high Plexiglas rim surrounded the open arms. The open field was a round arena (60 cm2), with the floor divided into 12 parts, and walls that were 50 cm high. Luminosity at the level of the T-maze arms or at the open field center was 60 lx. After the experimental session conducted with each animal, the ETM and the open field were cleaned with a 10% ethanol solution. In the ETM, the escape behavior, which mimics panic-like behavior,2829 is induced by an ethologically relevant threatening stimulus: the exposure to an open and elevated space. In addition, inhibitory avoidance is considered a defensive reaction, which models generalized anxiety.2829
Drugs
A selective TrkB receptor agonist, 7,8-dihydroxyflavone (Tokyo Chemical Industry Co. Ltd., Tokyo, Japan),14 was dissolved in phosphate-buffered saline containing 50% dimethylsulfoxide (DMSO). 7,8-DHF or vehicle was injected intraperitoneally (i.p.) once daily from the time after the CMS treatment for four weeks until the end of the experiment. Rats were given the last dose of 7,8-DHF or vehicle 2 hours prior to sacrifice. The doses of 7,8-DHF employed in the current experiments were 5 and 20 mg/kg.
Experiment design
The animals were trained to consume 2% sucrose solution in a 1-hour test that was performed twice a week (Figure 1A). Following the two-week sucrose consumption training, the sucrose intakes of the rats were obtained to allow the division of the rats into control group and the CMS group with both groups having similar sucrose intakes. The CMS group was exposed to stress for 4 weeks and subsequently to stress in combination with 7,8-DHF or vehicle treatment for 4 consecutive weeks. During the 8-week CMS period, the rats were subjected to behavioral tests once a week (sucrose consumption test) and in week 0, 4, 6 (locomotor activity), 7 (forced-swimming test), and 8 (ETM) of CMS period. Two days after the CMS period ended, the animals were sacrificed and their blood and brain tissues were collected for biochemical analysis.

Behavioral effects of the 4 week CMS. A: The CMS protocol involved subjecting animals to 8 week CMS. After 4 week CMS, animals were treated with 7,8-DHF or vehicle injection. During 8 week CMS period, sucrose consumption test were carried out once a week, Locomotion activity tests in week 0, 4, and 6, Forced swimming test in week 7 and elevated T-maze in week 8. B: The effect of 4 week CMS on body weight gain (C) Consumption of 2 % sucrose solution in rats exposure to 4 week CMS versus control rats. D: Body weight gain in the CMS rats and control rats treated with vehicle, 5 and 20 mg/kg 7,8-DHF for three weeks. Values are presented as mean+SEM. *p<0.05, **p<0.01, ***p<0.001, the CMS group vs. control group. #p<0.05, ##p<0.01, ###p<0.001, compared within the CMS or control group.
Chronic mild stress
The animals were divided into two matched groups and placed in separate rooms. The CMS procedure was adapted from the procedure described elsewhere3031 with slight modifications. Briefly described here, all rats in the CMS group were housed in single cages and exposed to the following stressors in a randomized fashion throughout the 8 weeks experiment: 1) 24 h food deprivation; 2) 24 h water deprivation; 3) housing in a cage filled with an odor; 4) 45°cage tilt and overnight illumination; 5) 24 h soiled cage (200 mL water in 100 g sawdust bedding). Normal control rats were left undisturbed in their home cages.
Measurement of body weight
The body weight of each rat was measured weekly from week 0 (initial week) to week 8, but was never recorded after the food or water deprivation-stressor of the CMS procedure.
Locomotor activity
Locomotor activity was measured using a computerized automated activity monitoring system (MED Associates, Inc., St. Albans, VT, USA). The system included four plexiglass chambers (43×43×30 cm3) equipped with an I/R array of 16 photodetectors and corresponding light sources that emitted photobeams 3 cm apart and 4.5 cm above the chamber floor. Travel distance was recorded at the assigned intervals and was controlled by the MED Associates software.
Sucrose consumption test
The methods for sucrose consumption test used to operationally define anhedonia, was adapted from the procedure described previously32 with slight modifications. The animals were first habituated to consume a palatable sucrose solution (2%). The habituation period lasted 2 weeks. In this period, the sucrose test was made twice a week. Animals were food and water deprived 15 hours before the test, which was a 1-hour period with free access to a bottle of the sucrose solution.
Forced-swimming test
Forced-swimming test (FST) was performed between 9:30 A.M. and 1:00 P.M. Experiments were performed as described previously.33 Briefly described here, the rats were placed for 15 min into a 25 cm diameter×50 cm height plastic cylinder, which was filled with 20–25℃ water to a depth of 30 cm. The rats were then removed, dried, and returned to their home cage. They were placed again in the cylinders 24 h later, and then a 5-min swim test was conducted and videotaped. Immobility was defined as the minimum movement required to passively keep the animal's head above the water without other motions. Climbing was defined as the upward-directed movement of the forepaws against the wall. The results are expressed as the amount of time (in seconds) that the animals spent immobile and climbing during the 5-min test.
Serum corticosterone levels
The rats were killed between 0800 and 1100, during which time their blood samples from trunk vessels were collected into chilled tubes. Serum corticosterone was quantified by using a commercially available enzyme-linked immunosorbent assay Sandwich ELISA Kit (Caymanchem) according to the manufacturer's instructions. All samples were performed in duplicate.
Hippocampal BDNF levels
We choose to test BDNF levels in this brain region because numerous studies have suggested a link between BDNF in the hippocampus and stress-related depression-like behavior3435 or the therapeutic effect of chronic antidepressant treatment.3637 When the rats were killed, their brains were rapidly removed. Bilateral hippocampi were rapidly dissected and stored at -80℃. At the time of analysis, the samples were weighed, and BDNF was extracted as described previously.38 Two milliliters of lysis buffer (100 mM PIPES, 500 mM NaCl, 0.2% Triton X-100, 0.1% NaN3, 2% BSA, and 2 mM EDTA) containing freshly prepared protease inhibitors (200 µM PMSF, 0.3 µM aprotinin, and 10 µM leupeptin) were added to each sample. The samples were then sonicated by pulses at 1 s intervals for 15 s. An additional 1 mL of lysis buffer was added, and the samples were then resonicated. All homogenates were centrifuged at 16,000×g for 30 min at 4℃, and supernatants were removed and frozen at -20℃ until assay. The concentration of hippocampal BDNF was quantified using the BDNF Sandwich ELISA kit (Abnova). The tissue samples and serial dilutions of BDNF standards were loaded in duplicate onto a 96-well plate coated with primary antibodies. The plate was sealed and incubated for 90 mins at 37℃. After the wells were washed three times with 400 µL/well of PBS, biotinylated anti-rat BDNF monoclonal antibody was added for 1 h at 37℃. The plates were washed four times. Strepavidin-enzyme conjugate was added, and the plates were incubated for 30 mins. After further washing, tetramethylbenzidine chromagenic substrate was added and the reaction was stopped after 20 min at room temperature. The absorbance at 450 nm was measured with a plate reader, and the BDNF concentration in the tissue was assessed by comparing the values to the prepared standard curve.
Data analysis
All data were expressed as mean±S.E.M. The data obtained from various groups were statistically analyzed using two-way analysis of variance (ANOVA) followed by the post hoc Tukey's multiple comparison test. The value of p<0.05 was considered as statistically significant.
RESULTS
Depressive-like behavior induced by CMS in rats
After 4-week CMS, a CMS×time interaction [group×time: F(4, 255)=27.98, p<0.001] was observed for the change of body weight of the rats (Figure 1B). The CMS significantly decreased the rate of body weight gain over the 28 day procedure [group: F(1, 255)=5.74, p=0.019]. In addition, the stressed rats showed significantly lower locomotor activity than their control counterparts (p<0.05). In the comparison of sucrose consumption, there was a CMS×time interaction [group×time: F(1, 60)=7.15, p=0.01]. As can be seen in Figure 1C, the CMS rats showed significantly lower sucrose consumption than their control counterparts (p=0.004), suggesting that CMS modeled anhedonia-like behavior of depressive phenotype in rats.
Effects of treatment with 7,8-DHF on behavioral and biochemical parameters in stressed rats and controls
Body weight
After 3-week treatment with 7,8-DHF, two-way ANOVA revealed that there were no effects of the CMS condition or treatment with 7,8-DHF on body weight (Figure 1D).
Sucrose consumption test
After 1-week treatment with 7,8-DHF, a CMS×7,8-DHF interaction [group×drug: F(2, 60)=7.35, p=0.001] (Figure 2A) was observed. Post-hoc tests confirmed that in the stressed rats, the 7,8-DHF 20 mg/kg condition caused a greater increase in sucrose consumption than the 7,8-DHF 5 mg/kg and DMSO vehicle conditions (p<0.001).
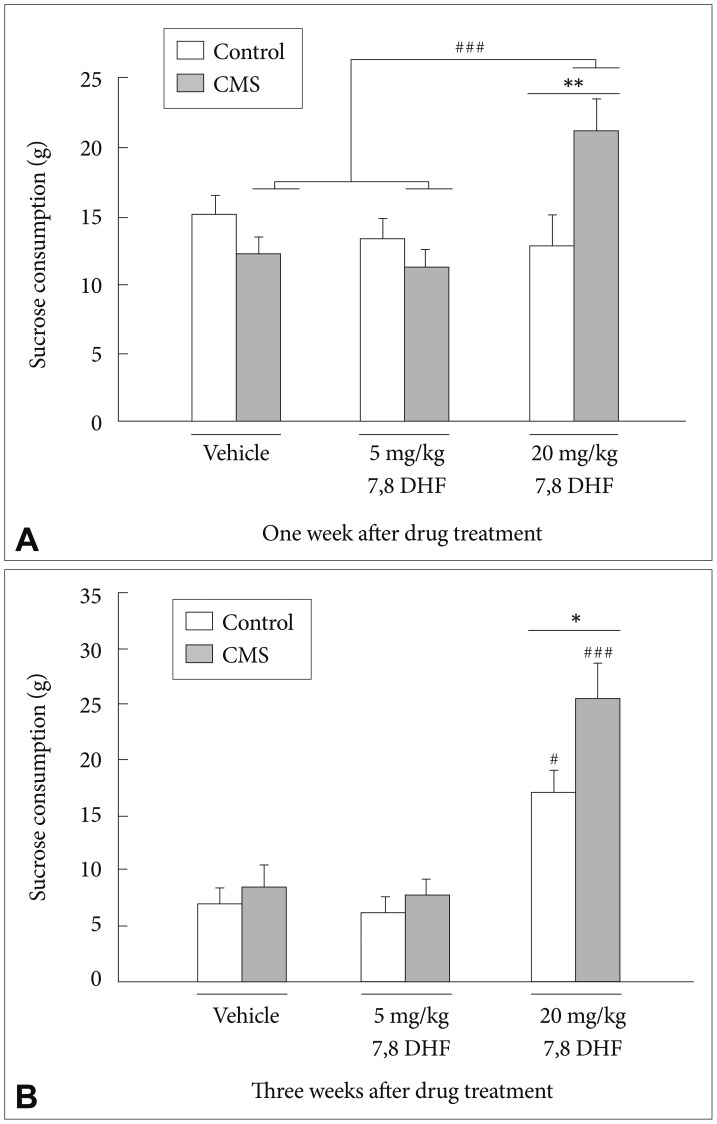
A: Consumption of 2% sucrose solution in the CMS rats and control rats treated with vehicle (n=11), 5 mg/kg (n=9) and 20 mg/kg (n=8) 7,8-DHF for one week and (B) for three weeks. Values are presented as mean+SEM. *p<0.05, **p<0.01, ***p<0.001, the CMS group vs. control group. #p<0.05, ##p<0.01, ###p<0.001, compared within the CMS or control group.
In addition, stressed rats treated with 7,8-DHF at 20 mg/kg had significantly greater sucrose consumption than their control counterparts (p<0.01). After 3-week treatment with 7,8-DHF, there were main effects of the CMS condition [group: F(1, 57)=5.94, p=0.018] and treatment with 7,8-DHF [drug: F(2, 57)=30.98, p<0.001] (Figure 2B). Post-hoc tests revealed that an injection of 20 mg/kg 7,8-DHF was able to significantly increase the sucrose consumption in both the stressed and control rats. In addition, the stressed rats treated with 7,8-DHF at 20 mg/kg had significantly greater sucrose consumption than their control counterparts (p<0.05).
Locomotor activity
Main effects of the CMS condition [group: F(1, 52)=10.60, p=0.002] and treatment with 7,8-DHF [drug: F(2, 52)=8.75, p<0.001] (Figure 3A) were shown in the locomotor activities of the rats. A subsequent analysis revealed that the control rats showed no difference in locomotion activity under either the DMSO vehicle or the 7,8-DHF conditions. Post-hoc tests confirmed that the stressed rats exhibited a greater locomotion activity under the 7,8-DHF 20 mg/kg condition than when under either the 7,8-DHF 5 mg/kg or DMSO vehicle conditions (p<0.01).
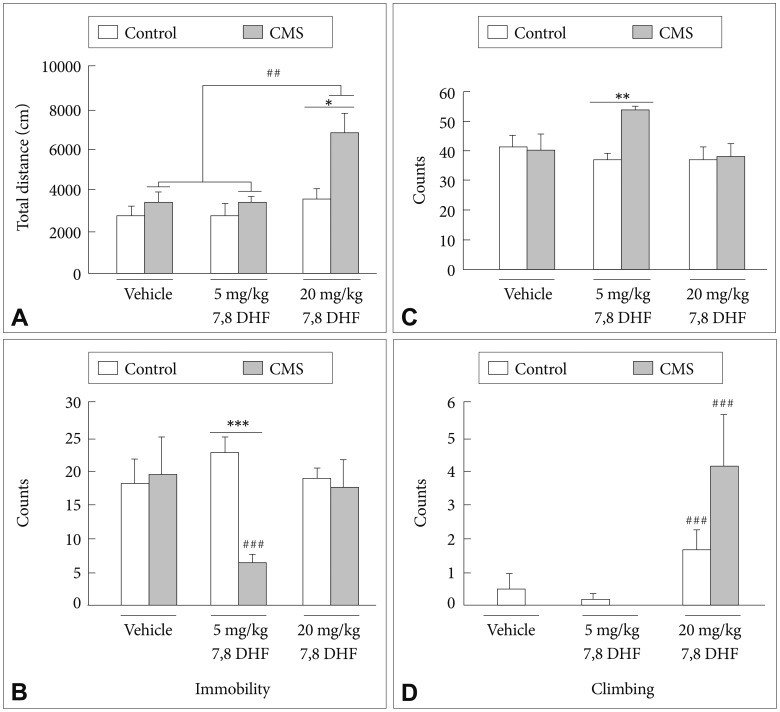
A: Locomotor activities in the CMS rats and control rats treated with vehicle and different 7,8-DHF dosages for 2 weeks. B: Immobility, C: swimming, and (D) climbing time in FST in the CMS rats and control rats treated with vehicle and different 7,8-DHF dosages for 3 weeks. The data represent the mean+SEM. *p<0.05, the CMS group vs. control group. ##p<0.01, compared within the CMS or control group.
Forced-swimming test
Regarding the immobility counts in the FST, a CMS×7,8-DHF interaction [group×drug: F(2, 58)=4.26, p=0.019] (Figure 3B) was observed. Post-hoc tests confirmed that in the stressed rats, the 7,8-DHF 5 mg/kg condition caused a greater reduction in the immobility counts than the 7,8-DHF 20 mg/kg and DMSO vehicle conditions (p<0.01). The stressed rats treated with 7,8-DHF at 5 mg/kg showed significantly lower immobility counts than their control counterparts (p<0.001). For the swimming counts in the FST, a CMS condition×7,8-DHF interaction [group×drug: F(2, 58)=4.61, p=0.014] (Figure 3C) was observed. The CMS rats treated with 7,8-DHF 5 mg/kg showed significantly higher swimming counts than their control counterparts (p<0.01). As for the climbing counts in the FST, a main effect of treatment with 7,8-DHF was shown [drug: F(2, 52)=8.75, p<0.001] (Figure 3D). Post-hoc tests revealed that an injection of 20 mg/kg 7,8-DHF was capable of significantly increasing the climbing counts in both the stressed and control rats (p<0.001).
Elevated T-maze test
In the baseline latencies measured in the ETM, there were no effects of the CMS condition [group: F(1, 53)=0.07, p=0.793] or treatment with 7,8-DHF [drug: F(2, 53)=1.89, p=0.162]. In the first avoidance (avoidance 1) latencies measured in the ETM, there was a CMS condition×7,8-DHF interaction [group×drug: F(2, 53)=3.61, p=0.034] with a main effect of treatment with 7,8-DHF [drug: F(2, 53)=9.38, p<0.001] (Figure 4A). The DMSO vehicle-treated stressed rats had significantly greater avoidance 1 latencies than their control counterparts (p<0.05). The stressed rats treated with 20 mg/kg rather than 5 mg/kg 7,8-DHF had significantly lower avoidance 1 latencies than those treated with DMSO vehicle (p<0.05). For the one-way escape latencies in the ETM, there were no effects of the CMS condition or treatment with 7,8-DHF (Figure 4B).
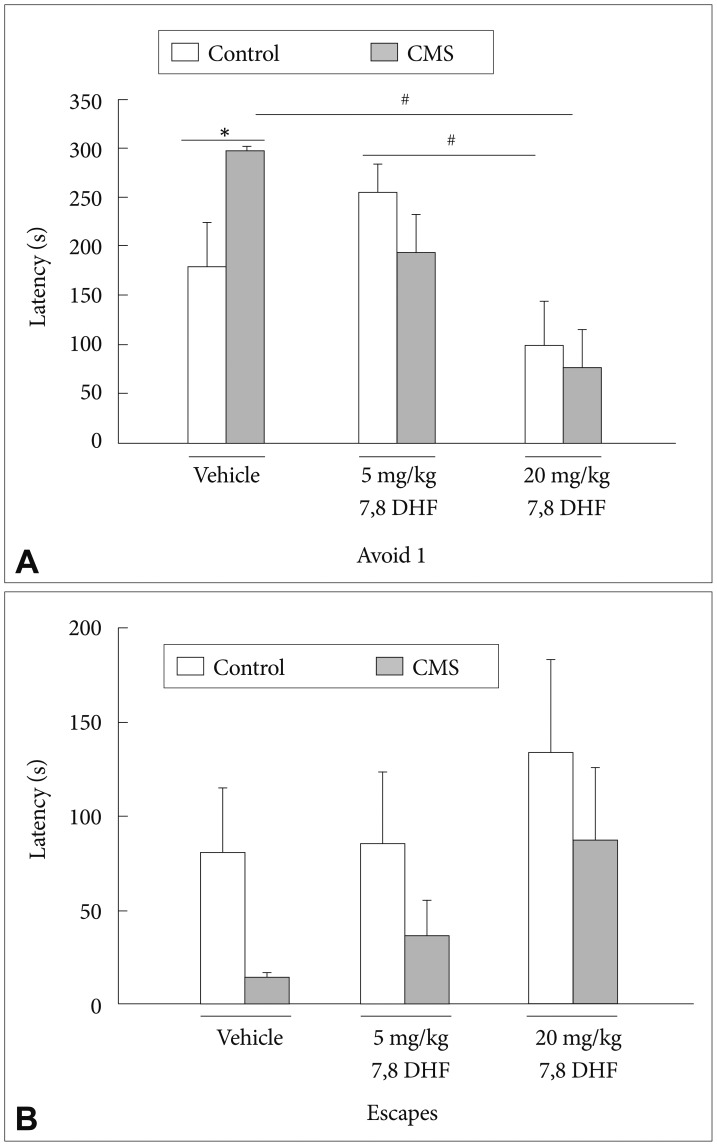
A: The latencies to leave the enclosed arm and (B) the open arm were measured from the CMS rats and control rats treated with vehicle and different 7,8-DHF dosages for 4 weeks. Result are presented as mean+SEM. *p<0.05, the CMS group vs. control group. #p<0.05, compared within the CMS or control group.
Hippocampal BDNF levels
In the hippocampal BDNF concentrations, there existed a main effect of the CMS condition [group: F(1, 12)=6.15, p=0.029] (Figure 5A). The DMSO vehicle-treated stressed rats had significantly lower BDNF levels than controls (p<0.01). In the stressed rats, the 7,8-DHF, when given at 5 mg/kg but not 20 mg/kg, significantly increased the BDNF levels. The control rats exhibited no significant difference in BDNF levels under either the DMSO vehicle condition or treatment with either doses of 7,8-DHF.
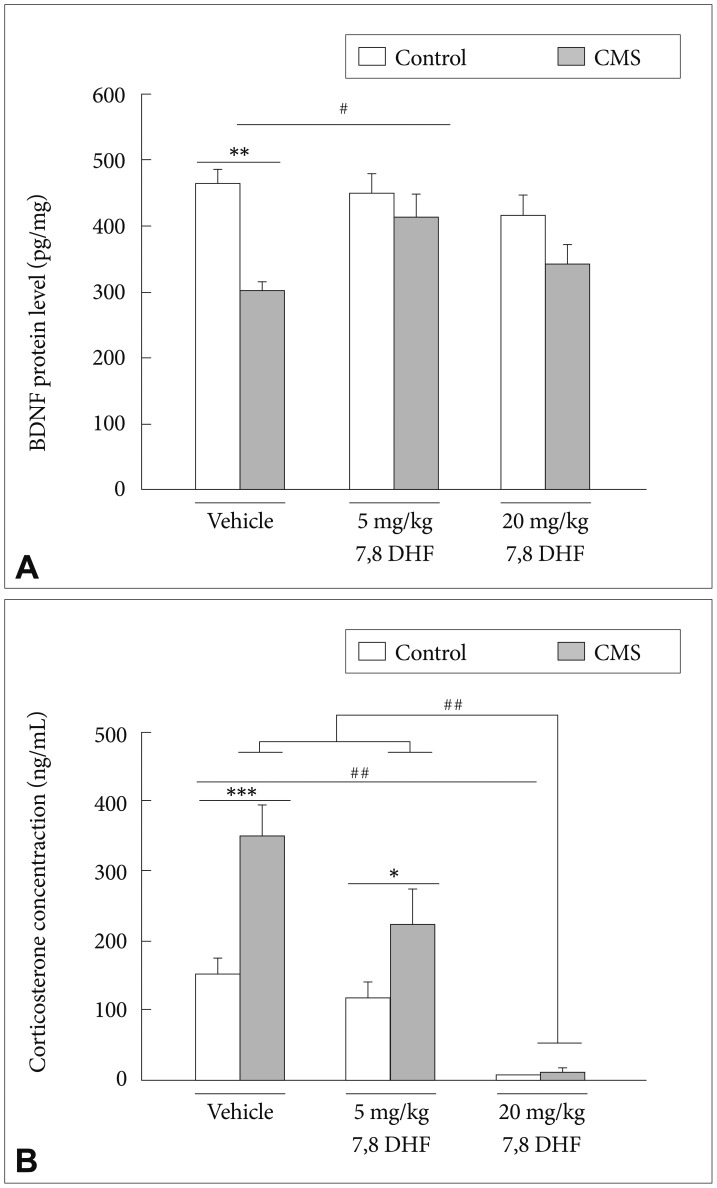
A: The BDNF protein level in hippocampus and (B) serum corticosterone level in the CMS rats and control rats treated with vehicle and different 7,8-DHF dosages for 3 weeks. Data represent mean+SEM. *p<0.05, **p<0.01, ***p<0.001, the CMS group vs. control group. #p<0.05, ##p<0.01, compared within the CMS or control group.
Serum corticosterone levels
In the serum corticosterone levels, there existed a CMS×7,8-DHF interaction [group×drug: F(2, 28)=5.26, p=0.011] with a main effect in the CMS condition [group: F(1, 28)=16.74, p<0.001)] (Figure 5B) and treatment with different doses of 7,8-DHF [drug: F(2, 28)=33.75, p<0.001]. The DMSO vehicle-treated stressed rats had significantly higher corticosterone levels than controls. 7,8-DHF dose-dependently reduced the corticosterone levels of the stressed rats, with greatest levels in the DMSO vehicle condition, intermediate levels in the 7,8-DHF 5 mg/kg condition, and lowest levels in the 7,8-DHF 20 mg/kg condition (p<0.001). For the control rats, the treatment with 20 mg/kg 7,8-DHF, compared to the treatment with the DMSO vehicle (p<0.05), was capable of significantly reducing the corticosterone levels.
DISCUSSION
In the present study, 7,8-DHF reversed the CMS-induced depressive-like and partial anxiety-like behaviors. Moreover, 7,8-DHF significantly increased the CMS-subjected rats' hippocampal BDNF levels and reduced their serum corticosterone levels, which may underlie the antidepressant and/or partial anxiolytic efficacy of the treatment.
The most striking finding of our study is that the CMS-induced anhedonia-like behavior was reversed after 7 days of the 7,8-dihydroxyflavone (20 mg/kg) treatment, and remained so across the subsequent treatment regime. In accordance with our finding, Zhang et al.39 even detected the effect of a single dose of 7,8-DHF to improve the decreased sucrose preference 1 day after administration in the social defeat stress model of depression. Conventional antidepressants that target monoaminergic systems and increase the synthesis of BDNF typically take several weeks to be effective. Although the time-lag between the drug administration and its therapeutic effect is not clearly understood, it may be partly explained by the BDNF-TrkB positive feedback mechanism, which is a protracted process.40 Specifically, BDNF binding to TrkB at synaptic sites leads to the internalization of the BDNF-TrkB complex, which is endocyted and retrograde transported from spines to cell bodies, where it activates the synthesis of more BDNF. By skipping this process and directly activating TrkB, 7,8-DHF may thereby produce a fast-onset antidepressant effect. This effect is also supported by a recent study reporting that the antidepressant effect of 7,8-DHF is in part mediated via mammalian target of rapamycin complex 1 (mTORC1) signaling,41 which is known to be implicated in the rapid antidepressant action of the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine.4243
We also noted the phenomenon that after repeated injections, the baseline of vehicle group has shifted, thus the difference between CMS and control rats become less apparent. The phenomenon has been reported previously,44 and is possibly due to the fact that the repeated injection itself (regarded as a predictable stress) may also change the hedonic state-this is particularly dominant in the already stressed rats (i.e., the CMS rats), which can be interpreted as a stress-coping over consumption. 44
Our study demonstrated that the administration of 7,8-DHF (5 mg/kg, i.p.) significantly reduced the despair behavior in the FST, without altering locomotor activity. An earlier report showed that chronic treatment of 7,8-DHF (5 mg/kg) for 21 days via oral administration evidently reduced the immobility in FST in mice.18 In a recent study using the social defeat stress model of depression, a single dose of 7,8-DHF (10 mg/kg, i.p.) attenuated an increased immobility time in depressed mice 3 hours after administration.39 These findings together with ours imply that 7,8-DHF could be a curative treatment for CMS induced depressive-like behaviors in rats. Interestingly, a recent study reported that intracerebroventricular infusion of 7,8-DHF (240 ng/day) served to completely prevent the rats from developing anhedonia after being exposed to the CMS protocol, 19 suggesting a role of 7,8-DHF in prophylactic treatment. Collectively, all these findings indicate the important role of BDNF-TrkB signaling in the both the action of antidepressants and the development of depression.2345
Unexpectedly, treatment with the larger dose of 7,8-DHF (20 mg/kg) had no effect on despair behavior. It is possible that this regime possesses psychostimulant effects, as evidenced by its ability to increase locomotor activity (Figure 3A), thus specifically increasing climbing behavior instead of immobility in the FST. It is also possible that, for reducing despair behavior, either the effective dose range of 7,8-DHF is narrow or there exists a reverse dose-dependent relationship. Taken together, 7,8-DHF being capable of effectively treating different parts of the depressive phenotype at different doses supports the idea that it has significant antidepressant potential. Future study is suggested to verify if 7,8-DHF at an intermediate dose (i.e. between 5 mg/kg and 20 mg/kg, i.p.) can simultaneously treat both anhedonia and despair behavior.
Significantly greater general anxiety-like behaviors were observed in the CMS-subjected rats compared to controls, which were not attributed to reduced locomotor activity as there was no between-group difference in locomotor activity. On the other hand, the CMS-subjected rats did not display a significant increase in panic-like behavior compared to controls. Previous studies have shown conflicting results related to the anxiety-like behavior in the CMS paradigm, such as no effect, 45 anxiogenic effect,46 and partial anxiolytic effect.47 The reason for the discrepancy may be attributed to the strain of the animal, condition of the test, and the duration of CMS exposure. Failure of the CMS paradigm to induce panic-like behavior in our stressed rats may result from high inter-individual variability in escape latencies measured in the ETM (Figure 4B). It is also possible that the duration of CMS exposure is not sufficient to generate a stable panic-like behavior in our stressed rats.
Although the treatment with 7,8-DHF at any dose has no effect on one-way escape (reflecting no panicolytic-like effect of this drug), 7,8-DHF at 20 mg/kg treatment in the stressed rats significantly reduced their avoidance latencies. Contrary to an earlier report demonstrating no effect of 7,8-DHF on rats'anxiety-like behavior on an elevated plus maze,27 our study is the first to provide evidence suggesting that 7,8-DHF may exert potential anxiolytic-like effect in stressed rats. However, this finding should be interpreted with caution because this drug at a dose of at 20 mg/kg also significantly increased locomotor activity; therefore we could not exclude the possibility that psychostimulant effects might be responsible for their behavioral effects on avoidance latencies measured in the ETM.
In accordance with a recent study reporting the CMS-induced biochemical alternations,48 the result of our study showed elevated serum corticosterone levels and decreased hippocampal BDNF levels in the CMS-subjected rats. The finding suggested an interplay between the two neurochemical parameters in the stressed rats and cohered with earlier reports describing HPA axis hyperactivation linking to low hippocampal BDNF level.4950 Specifically, stress-induced elevated corticosteroids have detrimental effects on regulating the function and structure of the hippocampus, possibly through alterations in the expression of hippocampal BDNF.51 For example, repeated corticosterone treatment resulted in a graded increase in depression-like behavior, which is associated with decreased hippocampal neurogenesis and BDNF levels.52 Interestingly, reduced hippocampal BDNF per se is not sufficient to mediate depression-like behavior,53 but resulted in a depressive phenotype when combined with stress exposure.54 It is known that hippocampal neurogenesis plays an important role in regulating depression-like behavior55 while stress-induced decreases in hippocampal neurogenesis have been proposed to contribute to the pathophysiology of depression.56 Thus, our finding that combined HPA axis hyperactivation and low hippocampal BDNF levels, possibly contributing to decreased hippocampal neurogenesis, provided an underlying mechanism for CMS paradigm to induce depressive phenotype in rats.
In the present study, administration of 7,8-DHF dose-dependently reduced the corticosterone levels and when given at 5 mg/kg, increased the hippocampal BDNF levels in the CMS-subjected rats. The elevation in the HPA functioning following the exposure to CMS might be the cause of the reduction in hippocampal BDNF levels;3552 treatment with 7,8-DHF probably restored the CMS-induced HPA axis alterations, which in turn might rescue the reduction in hippocampal BDNF levels.57 It is also possible that 7,8-DHF per se increases BDNF levels. Specifically, Wu et al.58 suggested that 7,8-DHF may trigger further BDNF production via a positive feedback mechanism, in which an initial enhancement of TrkB phosphorylation following 7,8-DHF treatment may activate the PI3K/Akt pathway, stimulating the synthesis of BDNF via cAMP response element-binding protein (CREB), which is an important transcription factor needed to regulate BDNF transcription. 59 The higher dose of 7,8-DHF (20 mg/kg), however, was less efficient in increasing the BDNF levels. It is possible that high concentrations of TrkB ligands resulted in a down-regulation of the TrkB response,60 and thus caused a decrease in the positive feedback mechanism. Another possibility is that the different pharmacological effects of flavonoids depend on their concentration.61 For example, Akt activation is induced by lower concentrations of the flavonoid quercetin and inhibited by higher concentrations.62 Collectively, the modulation of both HPA axis activity and BDNF levels may be responsible for the antidepressant efficacy of 7,8-DHF in the rat model of CMS. There is much data from animal studies pointing out promising results of 7,8-DHF to treat Alzheimer's disease,1517 Parkinson disease14 and PTSD.2526 Effects of neuroprotection, neurogenesis, synaptogenesis and normalization of BDNF-TrkB signaling were proposed action mechanisms in common. Together with our data indicate that 7,8-DHF may act as a powerful therapeutic tool for the treatment of various neuropsychiatric disorders including depression.
The current study was limited in the lack of phosphorylated TrKB receptor to fully confirm that 7,8-DHF activates TrkB signaling. Western blot or immunohistochemistry with p-TrkB and p-Akt antibodies should be carried out in the future study. Nevertheless, the effects of 7,8-DHF could be still confirmed by behavioral and neuroendocrinological indexes, as seen in the behavioral tests, serum corticosterone, and BDNF levels.
In conclusion, our study offers a novel therapeutic approach to depression: a TrkB agonist, 7,8-DHF. It possesses a fast-onset antidepressant effect and a partial anxiolytic effect. Most importantly, chronic systemic treatment with this drug has the benefit of being more feasible and less invasive than viral or recombinant protein delivery, and allows the chronic targeting of neural pathologies of depression where BDNF levels and HPA axis activity are dysregulated.
Acknowledgments
This study was supported in part by grants from the Ministry of Science and Technology of Taiwanese Government (MOST-103-2314-B-016-021) and the Tri-Service General Hospital Grant (TSGH-C104-128), and the National Defense Medical Research (MAB-104-007 and MAB-104-008).