|
 |
- Search
| Psychiatry Investig > Volume 8(3); 2011 > Article |
Abstract
Objective
Genetic variation in the serotonin-2C receptor encoded by the HTR2C gene is one of the genetic determinants of antipsychotic-induced weight gain. Peroxisome proliferator-activated receptors are nuclear receptors regulating the expression of genes involved in lipid and glucose metabolism. In this cross-sectional study, we investigated whether HTR2C-759C/T, HTR2C-697G/C, PPAR╬▒ V227A, and PPAR╬│ 161C/T genotypes were associated with metabolic syndrome (MetS) in patients with schizophrenia taking clozapine.
Methods
One hundred forty-six Korean patients using clozapine for more than one year were genotyped for the HTR2C-759C/T, HTR2C-697G/C, PPAR╬▒ V227A, and PPAR╬│ 161C/T polymorphisms, and their weight, waist circumference, blood pressure, triglycerides, high-density lipoprotein-cholesterol, total cholesterol, and glucose were measured. We used the criteria for MetS proposed by the National Cholesterol Education Program-adapted Adult Treatment Panel III.
Results
The prevalence of MetS was 47.3% and was similar among men (49%) and women (42.9%). We found no significant differences between patients with and without MetS in terms of genotypes or allele frequencies. Logistic regression analyses also revealed no association between MetS and each genotype.
Clozapine is known to cause the most severe metabolic adverse effect among antipsychotics. Clozapine appears to have the greatest potential to induce weight gain.1 In a naturalistic study, 36.6% of patients who treated with clozpine for at least 1 year were diagnosed with diabetes during the 5-year follow-up.2 In addition, clozapine has been linked to hypertriglyceridemia,3 hypercholesterolemia3,4 and associated with decrease in high-density lipoprotein (HDL) cholesterol.5 Long-term clozapine treatment has been associated with increased rates of hypertension.6
Metabolic syndrome (MetS) represents a constellation of cardiovascular risk factors that includes central obesity, dyslipidemia, hyperglycemia, and hypertension.7 Subjects with MetS face substantially increased risks for the development of diabetes8 and cardiovascular disease.9 Data obtained in various countries have shown that the prevalence of MetS in patients with schizophrenia ranged from 37% to 63% and that the relative risk for the MetS was 2-3 times greater among patients with schizophrenia than among the general population.10
Insulin resistance plays a key role as the pathogenesis of MetS.11 The current concept of the development of MetS is that environmental factors add to an underlying genetic preponderance to insulin resistance.12 The mechanism behind MetS in schizophrenia is not entirely clear. The high interindividual variability suggests that genetic make-up is a modulating factor.
Genetic variation in the serotonin 2C (5HT2C) receptor encoded by the HTR2C gene is one of the genetic determinants of antipsychotic-induced weight gain. Yuan et al.13 found that several polymorphisms in the promoter region of the HTR2C gene were associated with an increased risk of diabetes and obesity in patients with psychiatric disorders. Reynolds et al.14,15 and Ellingrod et al.16,17 found a positive association between the HTR2C-759C/T genotype and antipsychotic-induced weight gain. Mulder et al.18 found a positive association between the HTR2C-697G/C genotype and MetS in patients with schizophrenia.
Peroxisome proliferator-activated receptors (PPARs) are nuclear receptors regulating the expression of genes involved in lipid and glucose metabolism. The primary role of PPARs is to regulate the oxidation of fat within cells. PPARs increases fatty acid uptake into cells, enhance burning of fat by beta-oxidation, and activate uncoupling proteins19 which facilitates the disposal of fat in states of overabundance.
Excessive fatty acid storage is one of the mechanisms for insulin resistance. PPARs improve insulin sensitivity due to remove the excessive fatty acid from muscle. They are encoded by 3 distinct genes: PPAR╬▒, PPAR╬│, PPAR╬┤. PPAR╬▒ is mainly expressed in tissues in which fatty acid catabolism is important, such as the liver, kidney, heart, and muscle. The PPAR╬│ gene is highly expressed in adipose tissue, where it controls adipocyte differentiation and lipid storage, and modulates the action of insulin.
Several reports on the association between PPARs polymorphisms and metabolic disturbances have been published. One of the frequently occurring PPAR╬│ polymorphisms is C-to-T substitution in exon 6, which was identified in 1998 by Meirhaegue et al. and has been studied in relation to obesity, glucose intolerance, and cardiovascular risk.20-23 To date, only a few studies on the association of this polymorphism with MetS and its components have been conducted, and the results are controversial. Many polymorphisms of the PPAR╬▒ gene, especially the PPAR╬▒-V227A polymorphism in East Asians, have recently been described. This polymorphism has been studied in relation to the serum lipid concentration in the Asian population.24,25
A study conducted by Arulmozhi et al.26 showed that PPAR╬▒ and PPAR╬│ agonists significantly reversed the increase in triglycerides observed in response to antipsychotic drugs. These medications also reduced increases in insulin resistance and glucose levels for at least some of the antipsychotics.26
The objective of this cross-sectional study was to evaluate whether the polymorphisms HTR2C-759C/T, HTR2C-697G/C, PPAR╬│ 161C/T, and PPAR╬▒ V227A were associated with MetS in patients with schizophrenia taking clozapine.
This study was conducted from October 2007 to September 2008 at the outpatient and inpatient departments of Seoul National Hospital in Korea. The sample included patients diagnosed with schizophrenia who were 18-65 years of age and who had been taking clozapine for more than one year. All subjects had taken other antipsychotic medications before clozapine. The DSM-IV diagnosis27 was established by chart review, and no exclusion criteria for concomitant psychotropic or medical pharmacology were applied. All subjects provided written informed consent for the study procedures, which were approved by the institutional review board at Seoul National Hospital.
Data were collected on age, sex, duration of illness, current Clinical Global Impression-Severity score and number of cigarettes smoked daily. Data on the dosages and duration of current antipsychotics and the number of concomitant psychotropic medications were obtained from medical records. Information on the presence of and current medication regimen for diabetes, hypertension, and dyslipidemia was gathered via patient self-reports.
Body weight and height were measured using standard hospital scales and height measurement procedures while subjects wore light clothing without shoes. Body mass index (BMI) was calculated by weight (kg)/height (m2). Waist circumference was measured from the narrowest point between the lower border of the rib cage and the iliac crest after a modest expiration. Blood pressure was measured in the sitting position after a 10-min rest period. Fasting blood samples were taken in the morning after an 8-h overnight fast. Fasting plasma glucose, total cholesterol, triglycerides, and HDL cholesterol were measured in a central certified laboratory.
The present study used the criteria for MetS proposed by the National Cholesterol Education Program adapted Adult Treatment Panel III (ATP IIIA),28 which defined this condition as the presence of three or more of the following risk factors: central obesity; hypertriglyceridemia, with fasting plasma triglycerides of Ōēź150 mg/dL; low HDL cholesterol with fasting HDL cholesterol of <40 mg/dL in men and <50 mg/dL in women; hypertension, with systolic and/or diastolic blood pressure of Ōēź130/85 mmHg or known treatment for hypertension; and hyperglycemia, with fasting plasma glucose of Ōēź100 mg/dL or known treatment for diabetes. We used the definition of abdominal obesity for Asian populations: Ōēź90 cm in men and Ōēź80 cm in women from the Western Pacific regional office of the World health organization.29
Approximately 5 mL of venous blood was collected from each subject in an ethylenediaminetetraacetic acid tube. Both HTR2C-759C/T (rs3813928) and HTR2C-697G/C (rs518147) polymorphism were genotyped by the sequencing method and both PPAR╬│ 161C/T (rs3856806) and PPAR╬▒ V227A (rs2016520) were analyzed by the SNaPshot assay.
Genomic DNA was prepared from peripheral blood samples using a nucleic acid isolation device, QuickGene-mini80 (FUJIFILM, Tokyo, Japan). The Polymerase chain reaction (PCR) method was used to amplify those fragments by using UCSC In-Silico PCR (http://genome.ucsc.edu/cgi-bin/hgPcr?command=start). The final volume of the PCR was 10 mL, consisting of 10 ng of DNA, 0.0005 mM of each primer pair, 0.25 mM dNTPs, 3 mM MgCl2, 1 mL 1├Śreaction buffer, and 0.25U Taq DNA polymerase (Intron Biotechnology, Seongnam-Si, Gyeonggi-do, Korea). To amplify exon 1, the 500 mM of betaine was added to the PCR system, which was different with exons 2, 3, and 4. The PCR conditions used were as follows: initial denaturation at 94Ōäā for 5 min, followed by 35 cycles of denaturation at 94Ōäā for 30 s, annealing at 60-65Ōäā for 30 s, initial extension at 72Ōäā for 30-60 s, and final extension at 72Ōäā for 10 min. The PCR products were purified using a MultiScreen384-PCR Filter Plate (Milipore, Billerica, MA, USA). The purified products were then sequenced using a BigDye Terminator Cycle Sequencing Kit and an ABI 3730xl automated sequencer (Applied Biosystems, Foster City, CA, USA). The sequencing primers were the same as those used for the PCR amplification. Mutation analyses were performed using Phred, Phrap, Consed, Polyphred 5.04 software (http://droog.mbt.washington.edu/PolyPhred.html).
Genomic DNA was extracted using a SNaPshot Multplex kit (Foster City, CA, USA). The SNaPshot assay was performed according to the manufacturer's instructions (ABI PRISM SNa-PShot Multiplex kit, Foster City, CA, USA). Analysis was carried out using Genemapper software (version 3.0; Applied Biosystems).
Demographic and clinical characteristics of subjects were summarized using a descriptive procedure. All continuous variables were presented as means with standard deviations; they were compared between two groups using an independent t-test. Group differences in categorical variables were examined using the chi-square test.
The genotype distribution was tested for Hardy-Weinberg equilibrium by HAPANALYZER software (available at http://hap.ngri.go.kr/)30 based on chi-squared analysis, prior to association analyses. Associations between each polymorphism and MetS were analyzed using a multiple logistic regression analysis adjusted for potential confounding effects of age, sex, type of antipsychotics, dosage and duration of clozapine, presence of mood stabilizers (valproate, lithium, topiramate) and number of cigarettes smoked daily. Common allele was considered as a reference. A p-value of <0.05 was considered to be statistically significant. We used the SPSS 12.0 version for Windows (SPSS Inc., Chicago, IL, USA) for analyses.
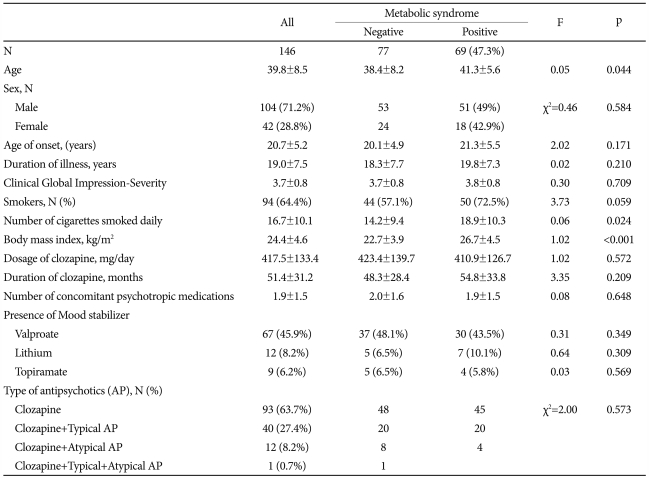
Of the total sample of 146 patients, 104 (71.2%) were men. All patients were of Korean ethnicity. The mean age was 39.8┬▒8.5 years (range: 21-62 years), and the mean duration of illness was 19.0┬▒7.5 years (range: 3-37 years). The mean clozapine dosage was 417.5┬▒133.4 mg/day, and the mean duration of clozapine use was 51.4┬▒31.2 months. The mean age of onset was significantly younger in male than in female patients (19.9┬▒4.9 vs. 22.6┬▒5.6, p=0.005) and the mean clozapine dosage was higher in male than female patients (432.3┬▒140.1 vs. 381.0┬▒108.3, p=0.035). More than half (63.7%) had been taking a clozapine for more than one year and the rest had been taking other antipsychotics with clozapine for more than one year. More than half (64.4%) of the sample currently smoked, and the smoking rate was significantly higher in men than in women (76% vs. 35.7%, Žć2=21.1, p<0.001).
According to the ATP IIIA, the prevalence of MetS among these patients was 47.3% and was similar among men (49%) and women (42.9%). When comparing with patients without MetS, patients with MetS were significantly older (41.3┬▒5.6 vs. 38.4┬▒8.2, p=0.044) and had significantly higher number of cigarettes smoked per day (18.9┬▒10.3 vs. 14.2┬▒9.4, p=0.024) and higher BMI (26.7┬▒4.5 vs. 22.7┬▒3.9, p<0.001)(Table 1).
All genotypes were in Hardy-Weinberg equilibrium with non-significant Žć2 values with respect to comparisons between the observed and expected genotype frequencies of each of the tested polymorphisms [(-759C/T)(p=0.215), (-697G/C)(p=0.198), (161C/T)(p=1.0), (V227A)(p=1.0)]. No significant differences in genotypes or allele frequencies between patients with and without MetS were observed (Table 2). Logistic regression analyses also showed no associations between MetS and each genotype (Table 3).
This study found no association between four polymorphisms (HTR2C-759C/T, HTR2C-697G/C, PPAR╬▒ V227A, and PPAR╬│ 161C/T) and MetS in patients with schizophrenia taking clozapine for more than 1 year.
Mulder et al. reported that several HTR2C polymorphisms were associated with MetS in patients taking various antipsychotics. In one report, the HTR2C-697G/C polymorphism was associated with MetS,18 but no significant association was found in a study attempting to replicate these results.31 Consistent with the results of our study, previous studies have shown that the HTR2C-759C/T polymorphism, which is known to be associated with antipsychotic-induced weight gain,14-16 had no association with MetS.18,31,32 Another polymorphism of HTR2C (rs1414334) has been reported to be significantly associated with MetS in patients with schizophrenia,18,31,32 and this association has been particularly strong in patients using clozapine and risperidone, which have both high affinity for the 5-HT2C receptor.31 All subjects enrolled in our study had been taking clozapine; despite this methodological advantage, the -697G/C and -759C/T polymorphisms of HTR2C had no significant association with MetS. In another study, the -697G/C and -759C/T polymorphisms of HTR2C were not associated with levels of insulin, triglycerides, and cholesterol in patients treated with olanzapine or clozapine.33
The common polymorphisms of PPAR, PPAR╬▒ V227A, and PPAR╬│ 161C/T, also had no significant association with MetS in patients with schizophrenia. The presence of the A227 allele was associated with lower serum concentrations of total cholesterol and triglycerides in women, but not in men.25 Our study did not include a sufficient number of female patients, and only two female participants had the C allele of V227A. Other reports have described the association of the PPAR╬▒ V227A polymorphism with serum lipid concentrations according to age, drinking habits, and exercise status in Japanese individuals,24,34 but we did not consider exercise and dinking status. One previous study reported that the PPAR╬│ 161C/T polymorphism had no association with the overall prevalence of MetS, but that a decrease in the HDL-cholesterol component was less prevalent in normal female subjects with the T allele.35 In our study, relatively few female patients had the T allele of 161C/T (n=11).
The association between PPAR polymorphisms and metabolic disturbances has been investigated primarily in normal populations or patients with diabetes. To date, only one study on PPAR polymorphisms in patients with schizophrenia has been published, and this study focused on the association of olanzapine-induced weight gain and PPAR╬│ Pro12Ala polymorphism in patients with schizophrenia.36 Our study is the first to focus on the association of PPAR polymorphisms with MetS in patients with schizophrenia. Although PPAR polymorphisms have not been associated with the prevalence of MetS, the association between individual metabolic components and these polymorphisms needs to be investigated.
Several reports on the prevalence of MetS in Korean patients with schizophrenia have emerged,37,38 and the present study is the first to investigate the prevalence of MetS in subjects who were treated with one kind of antipsychotic medication, clozapine. We found that 47.3% of the Korean patients with schizophrenia taking clozapine met the criteria for MetS, which is comparable to the prevalence rates reported in studies of MetS among European (50%)39 and US (53.8%) patients with schizophrenia taking clozapine.40 We thus confirmed the high prevalence of MetS in patients with schizophrenia taking clozapine.
There are several limitations in this study. First, the sample was relatively small; thus, a more extended study with a larger population is needed. Second, because this study used a cross-sectional design, we could not evaluate the causal relationships between MetS and clozapine. Data on metabolic parameters of the patients at the initiation of clozapine treatment were not available to us. Therefore it was not possible to analyze data for change in these parameters over time related to the use of clozapine. Henderson et al.2 reported that clozapine-induced weight gain continued until approximately 46 months following initiation of clozapine treatment, after which weight gain appeared to level off. Therefore the duration of the clozapine treatment in this study (51.4 months) may have been sufficient to determine the metabolic adverse effects of this medication. Third, several variables other than genetic factors can contribute to the risk of MetS. Although we considered the factors that are typically associated with potential influence on metabolic states, such as smoking and mood stabilizers, we failed to include other parameters, such as physical activity, diet and other medications which might cause weight gain.
Our results show that the HTR2C-759C/T, HTR2C-697G/C, PPAR╬▒ V227A, and PPAR╬│ 161C/T SNPs were not associated with MetS in patients with schizophrenia taking clozapine. The possibility that these SNPs may serve as risk markers for metabolic adverse effects should be the subject of further examination.
Acknowledgments
This study was supported by the Choi Shin Hae Korean Foundation of Neuropsychiatric Research. We are particularly grateful to Ka Hee Lee, Gwon Young Kang, Kyong Hoon Kim, Kwon Kon Kim, Minah Soh, Min Young Sim, Hwang Bin Lee for helping with data collection and to Tae Kyung Lee, Hae Ree Han, Dong Yeon Park for advising on study design and analyses.
References
1. Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-1696. PMID: 10553730.


2. Henderson DC, Cagliero E, Gray C, Nasrallah RA, Hayden DL, Schoenfeld DA, et al. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities : A five-year naturalistic study. Am J Psychiatry 2000;157:975-981. PMID: 10831479.


3. Wu RR, Zhao JP, Liu ZN, Zhai JG, Guo XF, Guo WB, et al. Effects of typical and atypical antipsychotics on glucose-insulin homeostasis and lipid metabolism in first-episode schizophrenia. Psychopharmacology (Berl) 2006;186:572-578. PMID: 16601995.


4. Lindenmayer JP, Czobor P, Volavka J, Citrome L, Sheitman B, McEvoy JP, et al. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. Am J Psychiatry 2003;160:290-296. PMID: 12562575.


5. Wirshing DA, Boyd JA, Meng LR, Ballon JS, Marder SR, Wirshing WC. The effects of novel antipsychotics on glucose and lipid levels. J Clin Psychiatry 2002;63:856-865. PMID: 12416594.


6. Henderson DC, Daley TB, Kunkel L, Rodrigues-Scott M, Koul P, Hayden D. Clozapine and hypertension : a chart review of 82 patients. J Clin Psychiatry 2004;65:686-689. PMID: 15163256.


8. Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes 1992;41:715-722. PMID: 1587398.


9. Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001;24:683-689. PMID: 11315831.


10. Correll CU. Balancing efficacy and safety in treatment with antipsychotics. CNS Spectr 2007;12(10 Suppl 17):12-20. 35PMID: 17934385.


11. Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002;288:2709-2716. PMID: 12460094.



12. Hong Y, Pedersen NL, Brismar K, de Faire U. Genetic and environmental architecture of the features of the insulin-resistance syndrome. Am J Hum Genet 1997;60:143-152. PMID: 8981957.


13. Yuan X, Yamada K, Ishiyama-Shigemoto S, Koyama W, Nonaka K. Identification of polymorphic loci in the promoter region of the serotonin 5-HT2C receptor gene and their association with obesity and type II diabetes. Diabetologia 2000;43:373-376. PMID: 10768099.


14. Reynolds GP, Zhang ZJ, Zhang XB. Association of antipsychotic drug-induced weight gain with a 5-HT2C receptor gene polymorphism. Lancet 2002;359:2086-2087. PMID: 12086765.


15. Reynolds GP, Zhang Z, Zhang X. Polymorphism of the promoter region of the serotonin 5-HT(2C) receptor gene and clozapine-induced weight gain. Am J Psychiatry 2003;160:677-679. PMID: 12668355.


16. Ellingrod VL, Perry PJ, Ringold JC, Lund BC, Bever-Stille K, Fleming F, et al. Weight gain associated with the-759C/T polymorphism of the 5HT2C receptor and olanzapine. Am J Med Genet B Neuropsychiatr Genet 2005;134B:76-78. PMID: 15666332.


17. Miller DD, Ellingrod VL, Holman TL, Buckley PF, Arndt S. Clozapine-induced weight gain associated with the 5HT2C receptor-759C/T polymorphism. Am J Med Genet B Neuropsychiatr Genet 2005;133B:97-100. PMID: 15635667.


18. Mulder H, Franke B, van der-Beek van der AA, Arends J, Wilmink FW, Scheffer H, et al. The association between HTR2C gene polymorphisms and the metabolic syndrome in patients with schizophrenia. J Clin Psychopharmacol 2007;27:338-343. PMID: 17632216.


19. Luquet S, Lopez-Soriano J, Holst D, Gaudel C, Jehl-Pietri C, Fredenrich A, et al. Roles of peroxisome proliferator-activated receptor delta (PPARdelta) in the control of fatty acid catabolism. A new target for the treatment of metabolic syndrome. Biochimie 2004;86:833-837. PMID: 15589693.


20. Meirhaeghe A, Fajas L, Helbecque N, Cottel D, Lebel P, Dallongeville J, et al. A genetic polymorphism of the peroxisome proliferator-activated receptor gamma gene influences plasma leptin levels in obese humans. Hum Mol Genet 1998;7:435-440. PMID: 9467001.


21. Valve R, Sivenius K, Miettinen R, Pihlajamaki J, Rissanen A, Deeb SS, et al. Two polymorphisms in the peroxisome proliferator-activated receptor-gamma gene are associated with severe overweight among obese women. J Clin Endocrinol Metab 1999;84:3708-3712. PMID: 10523018.

22. Poulsen P, Andersen G, Fenger M, Hansen T, Echwald SM, V├Ėlund A, et al. Impact of two common polymorphisms in the PPARgamma gene on glucose tolerance and plasma insulin profiles in monozygotic and dizygotic twins: thriffy genotype, thriffy phenotype, or both? Diabetes 2003;52:194-198. PMID: 12502512.


23. Wang XL, Oosterhof J, Duarte N. Peroxisome proliferator-activated receptor gamma C161-->T polymorphism and coronary artery disease. Cardiovasc Res 1999;44:588-594. PMID: 10690291.



24. Naito H, Kamijima M, Yamanoshita O, Nakahara A, Katoh T, Tanaka N, et al. Differential effects of aging, drinking and exercise on serum cholesterol levels dependent on the PPARA-V227A polymorphism. J Occup Health 2007;49:353-362. PMID: 17951966.


25. Chan E, Tan CS, Deurenberg-Yap M, Chia KS, Chew SK, Tai ES. The V227A polymorphism at the PPARA locus is associated with serum lipid concentrations and modulates the association between dietary polyunsaturated fatty acid intake and serum high density lipoprotein concentrations in Chinese women. Atherosclerosis 2006;187:309-315. PMID: 16288935.


26. Arulmozhi DK, Dwyer DS, Bodhankar SL. Antipsychotic induced metabolic abnormalities : an interaction study with various PPAR modulators in mice. Life Sci 2006;79:1865-1872. PMID: 16828808.


27. American Psychiatric Association. Diagonstic and Statistical Manual of Mental Disorders. 1994,Fourth Edition. Washington, DC: American Psychiatric Press.
28. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome : an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735-2752. PMID: 16157765.


29. World Health Organization. The Asia-Pacific Perspective: Redefing Obesity And Its Treatment. 2000,Sydney: Health Communication Australia; p. 15-21.
30. Jung HY, Park JS, Park YJ, Kim YJ, Kimm K, Koh IS. HapAnalyzer : minimum haplotype analysis system for association studies. Genomics Inform 2004;2:107-109.
31. Mulder H, Cohen D, Scheffer H, Gispen-de Wied C, Arends J, Wilmink FW. HTR2C gene polymorphisms and the metabolic syndrome in patients with schizophrenia: a replication study. J Clin Psychopharmacol 2009;29:16-20. PMID: 19142101.


32. Risselada AJ, Vehof J, Bruggeman R, Wilffert B, Cohen D, Al Hadithy AF, et al. Association between HTR2C gene polymorphisms and the metabolic syndrome in patients using antipsychotics: a replication study. Pharmacogenomics J 2010;in pressing.

33. Gunes A, Melkersson KI, Scordo MG, Dahl ML. Association between HTR2C and HTR2A polymorphisms and metabolic abnormalities in patients treated with olanzapine or clozapine. J Clin Psychopharmacol 2009;29:65-68. PMID: 19142110.


34. Naito H, Yamanoshita O, Kamijima M, Katoh T, Matsunaga T, Lee CH, et al. Association of V227A PPARalpha polymorphism with altered serum biochemistry and alcohol drinking in Japanese men. Pharmacogenet Genomics 2006;16:569-577. PMID: 16847426.


35. Rhee EJ, Oh KW, Lee WY, Kim SY, Oh ES, Baek KH, et al. Effects of two common polymorphisms of peroxisome proliferator-activated receptor-gamma gene on metabolic syndrome. Arch Med Res 2006;37:86-94. PMID: 16314192.


36. Herken H, Erdal M, Aydin N, Sengul C, Karadag F, Barlas O, et al. The association of olanzapine-induced weight gain with peroxisome proliferator-activated receptor-gamma2 Pro12Ala polymorphism in patients with schizophrenia. DNA Cell Biol 2009;28:515-519. PMID: 19622037.


37. Nam YY, Kim CS, Ahn CW, Park KM, Ryu B, Kim CH. Clinical correlates of metabolic syndrome in patients with chronic schizophrenia. Korean J Psychopharmacol 2006;17:335-341.
38. Jeong JT, Yoon BH, Kim TU, Sea YH, Park SH, Jeong KY, et al. Prevalence of metabolic syndrome in chronic schizophrenic inpatients. Korean J Schizophr Res 2009;12:83-89.
39. De Hert M, Schreurs V, Sweers K, Van Eyck D, Hanssens L, Sinko S, et al. Typical and atypical antipsychotics differentially affect long-term incidence rates of the metabolic syndrome in first-episode patients with schizophrenia: a retrospective chart review. Schizophr Res 2008;101:295-303. PMID: 18299188.


40. Lamberti JS, Olson D, Crilly JF, Olivares T, Williams GC, Tu X, et al. Prevalence of the metabolic syndrome among patients receiving clozapine. Am J Psychiatry 2006;163:1273-1276. PMID: 16816234.


Table┬Ā2
Comparison of genotype and allele frequencies of polymorphisms with presence of metabolic syndrome

Table┬Ā3
Logistic analysis of polymorphisms with the risk of metabolic syndrome

*data were adjusted for age, sex, type of antipsychotics, dosage and duration of clozapine, presence of mood stabilizers (valproate, lithium, topiramate) and number of cigarettes smoked per day, ŌĆĀdata were analyzed with the common genotype as the reference for all polymorphisms. SNP: single nucleotide polymorphism, CI: confidence interval, OR: odds ratio