Electroencephalographic Abnormalities in Clozapine-Treated Patients: A Cross-Sectional Study
Article information
Abstract
The objective of our study was to examine the electroencephalogram (EEG) abnormalities associated with clozapine treatment. It was a cross-sectional study on 87 psychiatric patients on clozapine treatment. 32 channel digital EEG was recorded and analysed visually for abnormalities. EEG abnormalities were observed in 63.2% of patients. Both slowing and epileptiform activities were noted in 41.4% of patients. The EEG abnormalities were not associated with dose or duration of clozapine exposure.
INTRODUCTION
Clozapine is a dibenzodiazepine derivative which has receptor blocking activity at dopamine D1 and D4 as well as serotonin 5-HT1A and 5-HT2 receptors.1,2 It is an atypical antipsychotic that has been shown to be effective in treating patients with refractory schizophrenia.3 Clozapine also is less likely to cause extrapyramidal adverse effects including tardive dyskinesia than other antipsychotics.4 However, it has propensity to cause severe adverse effects such as agranulocytosis (1-2%)5 and an unusually high incidence of seizures.6,7
Recent estimates of incidence of clozapine-induced seizures vary from 1.3%8 to 2.8%.9 Even higher occurrence rates of seizures have been reported in more selected populations such as state hospital patients10 or brain injured patients.11 There is evidence that the risk for seizures is dose-related,7,12 although seizures can occur in all dose ranges10 and even at very low doses.13
Electroencephalogram (EEG) abnormalities have been reported to occur frequently in several retrospective studies of patients treated with clozapine;14-18 it ranges from 53%17 and 59%15 to 74%.18 Generalized slowing has been reported as the most prominent finding, followed by epileptiform activities such as spike-waves and sharp activities.15,17-19 Several but not all studies found EEG changes associated with clozapine to be dose-related.17,18,20 Furthermore, studies have shown that the plasma levels of clozapine predict pathological EEG changes as well as the occurrence of seizures.21,22 Some of these seemingly contradictory observations might be explained by clozapine's wide range of plasma level for a given dose.21 Volavka et al.23 reported that clozapine differed from the other antipsychotics by producing more EEG slowing and epileptiform activity. The aim of our study was to explore and characterize the EEG abnormalities associated with clozapine treatment.
METHODS
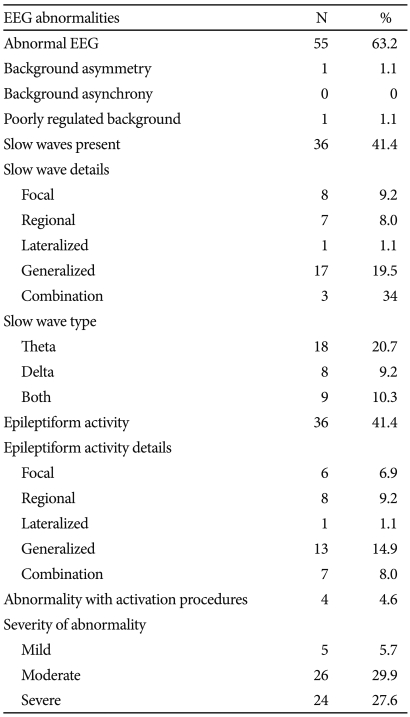
This was a cross-sectional study conducted at Center for Cognitive Neurosciences, Central Institute of Psychiatry, Ranchi, India. Sample consisted of 87 patients on clozapine treatment for various psychiatric disorders. All the patients were receiving clozapine for at least two weeks. Patients with history of seizures in the recent past were excluded from the study. Digital EEG was recorded in awake state with eyes closed using 21 Ag-AgCl electrodes (32 channels) placed according to the international 10-20 system using Neurofax EEG-9000 (Nihon Kohden, Japan) amplifiers (sampling rate 512 Hz, TC 0.1 Sec., HFF 70 Hz) with linked ear electrodes as reference. Three minutes of hyperventilation as well as photic stimulation in different frequencies were also obtained. Visual analysis of the EEG was carried out by the author NG, having experience in EEG interpretation after appropriate training, to characterize background activity as well as to identify slow waves and epileptiform activity (spike-waves, polyspike-waves or sharp waves) and their localization. EEG abnormalities were categorized on the basis of duration of abnormalities in 95 to 100 epochs each of 10 seconds duration in real time that were characterized as 'mild' (<25% of epochs), 'moderate' (25-50% of epochs), and 'severe' (>50% of epochs or polyspikes).
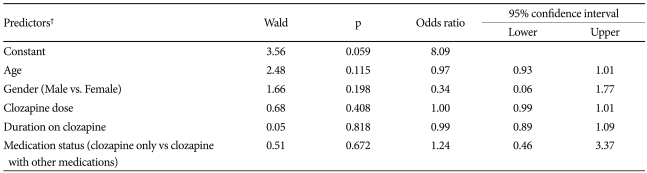
The data obtained were analyzed with Statistical Package for Social Sciences-version 10.0 for Windows® (SPSS Inc., Chicago, IL, USA). Continuous variables were analyzed using independent sample 't' test and categorical data was analyzed using Pearson's chi-square test. Level of significance was set at p<0.05 (two-tailed). Logistic regression analysis (backward conditional method) was carried out with presence or absence of EEG abnormality as outcome variable. Age, gender, clozapine dose, duration on clozapine and medications were entered as predictor variables.
RESULTS
Sample consisted of 76 (87.4%) males and 11 (12.6%) females. Mean age of the sample was 32.76 (SD 11.10) years and mean duration of illness was 92.39 (SD 64.79) months. Entire sample was right handed. Daily clozapine doses varied from 25 to 800 (mean 293.10, SD 172.55) mg/day and mean duration on clozapine was 15.07 (SD 8.98) weeks. Fifty-six (64.4%) patients were on clozapine alone, whereas 19 (21.8%) received valproate, 7 (8%) other antipsychotics and 5 (5.7%) antidepressants along with clozapine. Sixty-three (72.4%) had schizophrenia, 9 (10.3%) had mood disorders and 6 (6.9%) had schizoaffective disorder. Five (5.7%) patients had past history of epilepsy during childhood and three (3.4%) patients had head injury or central nervous system infection. EEG abnormalities associated with clozapine are summarized in Table 1. There was no difference in clozapine dose between patients with or without EEG abnormalities (t=0.96, p=0.342). Abnormal EEG was found in 34 (66.7%) patients receiving clozapine more than 300 mg/day, whereas it was seen in 21 (58.3%) patients receiving clozapine less than 300 mg/day. Among those receiving clozapine 100 mg or less per day (n=21), EEG was abnormal in 13 (61.9%) patients, whereas in those receiving clozapine 50 mg or less per day (n=12) and 25 mg or less per day (n=7), abnormal EEG was found in 6 (50%) and 4 (57.1%) patients, respectively. Abnormal EEG was found in 38 (67.9%), 11 (57.9%), 3 (42.9%) and 3 (60%) patients, among those receiving clozapine only, clozapine with sodium valproate, clozapine with antipsychotics and clozapine with antidepressants, respectively.
There was no difference in the frequency of EEG abnormalities (χ2=1.45, p=0.228), presence of slow waves (χ2=0.14, p=0.707) or epileptiform activities (χ2=0.69, p=0.406) among patients receiving clozapine alone or clozapine with other drugs. There was no significant difference in age, sex, duration of illness and duration on clozapine treatment between the patients with or without EEG abnormalities (Table 2). Thirty-eight (69.1%) patients out of 63 patients with schizophrenia had abnormal EEG, although it was not significantly higher than patients with other diagnoses (χ2=0.83, p=0.36). None of the patients developed clinical seizures. Logistic regression analysis did not identify any significant predictors of EEG abnormalities (Table 3).
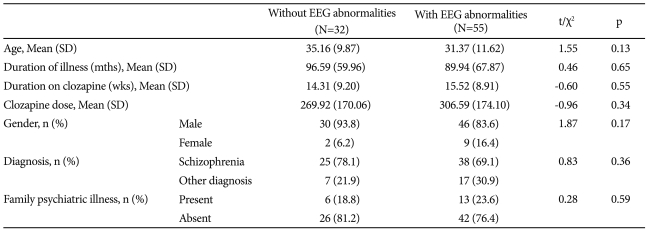
Comparison of clinical characteristics between those with and without EEG abnormalities associated with clozapine
DISCUSSION
The overall frequency of EEG abnormality was high (63.2%) in our study, that is consistent with the existing literature. EEG abnormalities occurred in 66.7% of patients receiving more than clozapine 300 mg/day; similar rates (72%) were reported earlier.14 Treves and Neufeld24 had found EEG abnormalities in 64% of patients under clozapine at 300 mg/day. Slowing of EEG in theta and delta range has been more frequent as com-pared to epileptiform activities in most studies,19 whereas, in our study both were equally prevalent (-40%).
In their retrospective review, Gunther et al.17 found a dose dependent relationship between clozapine dose and EEG slowing, with 92% of 283 patients receiving more than 600 mg clozapine exhibiting diffuse slowing. Liukkonen et al.25 reported background disturbance in 11, paroxysmal slow wave activity in 8, and epileptiform discharges in 4 out of 12 patients; patients with epileptiform discharges were on clozapine 300-700 mg daily. In our study no such association was found. Nevertheless, moderate to severe abnormalities were more frequent in our study. Interestingly, EEG abnormalities were present in more than 50% patients, even in those receiving lower doses of clozapine (less than 100, 50 or 25 mg per day). This finding underscores the importance of EEG monitoring in patients on lower doses of clozapine. However, these EEG abnormalities are not specific and the epileptiform activity is not necessarily associated with seizures, as observed in our study.
Quantitative EEG studies have reported an increase in theta and delta power and coherence changes in theta band following clozapine treatment.26 In that study, changes of coherence were correlated with changes on the Brief Psychiatric Rating Scale score, thus to clinical improvement. In a case study, sharp-slow wave activity was found bilaterally over frontal regions, and further source analysis localized these to the deep medial frontal region.27 Further studies are required to characterize these EEG changes prospectively.
The mechanism of clozapine-induced seizure is not known, although its effect on gamma-aminobutyric acid A (GABAA) receptors28 has been implicated. The other possible mechanisms include its action on nicotinic acetylcholine receptors,29 glutamate N-methyl-D-aspartate (NMDA) receptors,3 0 serotonin 5-HT2A31 and strychnine-sensitive glycine receptors33 that merits further evaluation. In a mouse model, expression of immediate early gene such as c-fos has been found in number of subcortical sites and in orbital cortex of clozapine kindled myoclonic jerks.33
Recently it has been suggested that EEG findings should not be excessively relied upon as there is no consensus on using EEG data as a guide for optimal dosing of clozapine.34 Several studies35,36 including ours, show that EEG abnormalities may be present without any association with clinical seizures, in contrast to the study by Welch et al.18 which suggested that the EEG is a sensitive indicator of clozapine toxicity and that changes such as the development of spikes and sharp waves indicate a high risk of convulsions. Larger studies are required to clarify this issue further.
In our cohort of 87 patients, EEG changes during clozapine therapy were common. Epileptiform activity was found in more than 40% of the patients and was of moderate to severe degree. However, in absence of baseline EEG, the issue of new onset abnormalities could not be clarified. Other limitation in our study includes heterogeneity of psychiatric diagnosis, the effect of which could not be examined due to small sample size. Also, serum clozapine levels were not measured in our study. It has been found that there are significant inter- and intra-individual variations in clozapine serum levels, for a given dose, and it has been attributed to its complex metabolism.21,37 A recent review38 found that although there was evidence for strong relationship between clozapine dose and plasma level and occurrence of clozapine-induced EEG abnormalities, there was no statistically significant relationship between dose and occurrence of seizures. The authors concluded that there is insufficient data to suggest direct relation-ship of clozapine plasma level with occurrence of seizures, though they found three case reports suggesting very substantial risk of seizures with clozapine plasma levels exceeding 1,300 µg/L.38 Nevertheless, therapeutic drug monitoring of clozapine has been suggested to be useful to identify signs of toxicity and onset of seizures.37
It is also interesting to note that in Asian patients, the plasma clozapine levels were higher with a given dose of clozapine, compared to Caucasian patients, and the findings remained significant even after controlling for gender, body mass index, cigarette, alcohol and caffeine use.39 Although ethnic differences in EEG abnormalities and seizures associated with clozapine has not been adequately studied, such variations might account for the differences in rates of EEG abnormalities. Two case studies40,41 described seizures occurring at lower clozapine doses in Asian patients, with very high serum levels in one report. In our study, frequent EEG abnormalities in those receiving lower doses of clozapine could reflect such ethnic variation, though such association is presumptive at best in the absence of plasma levels.
EEG abnormalities have also been reported to occur with typical as well as other atypical antipsychotics. One study42 compared EEG abnormalities in those receiving antipsychotics (n=81) with normal healthy controls (n=30). Abnormal EEG was higher in those receiving olanzapine (35%) and haloperidol (23%) and was statistically significantly increased with dose in the olanzapine group, in contrast to patients treated with haloperidol, quetiapine or healthy subjects. Also, epileptiform activity was observed only in patients receiving olanzapine (11%). In another study43 on a larger sample (n=323), EEG abnormalities were most frequent with clozapine (47%), followed by olanzapine (38%), risperidone (28%), typical antipsychotics (14%) and none with quetiapine. In that study, comorbid disorders and older age were associated with higher risk, whereas there was no relationship with dose or clinical response.
There is some evidence to suggest a favorable clinical response in specific groups of patients (females, major depressive disorder) predicted by EEG abnormalities before clozapine treatment.44 In another study,45 pretreatment intrahemispheric asymmetry on EEG predicted short-term response to clozapine treatment in schizophrenia. Also, EEG abnormalities developing after clozapine treatment appeared to be associated with good response in another small study.46
There is no consensus regarding antiepileptic prophylaxis in patients on clozapine without clinical seizures; some reports38 advising use of these medications at a certain clozapine dose or plasma level (>500 µg/L) or appearance of clear epileptiform discharges on EEG. However, as shown in our report, seizures were absent in patients receiving higher clozapine doses, even in the presence of epileptiform discharges on EEG. Therefore, routine use of anticonvulsants may not be warranted in patients having EEG abnormalities, even in those having epileptiform discharges. Nevertheless, it appears prudent to monitor for the clinical appearance of seizures in these patients, specifically with higher doses.