|
 |
- Search
| Psychiatry Investig > Volume 9(2); 2012 > Article |
Abstract
Objective
Capsaicin, a noxious stimulant and main component of the hot flavor of red peppers, has an analgesic effect when administered to humans. We investigated the expression of proopioimelanocortin (POMC) mRNA in the arcuate nucleus of Sprague-Dawley (SD) rats after administering capsaicin, hypothesizing that administering capsaicin activates the central opioid system.
Methods
SD rats were divided randomly into two groups; one group received a saline injection and the other received a capsaicin injection. The POMC mRNA level in the arcuate nucleus of the hypothalamus was measured by the reverse transcription-polymerase chain reaction at 0, 20, 40, 60, and 120 minutes after capsaicin administration.
Results
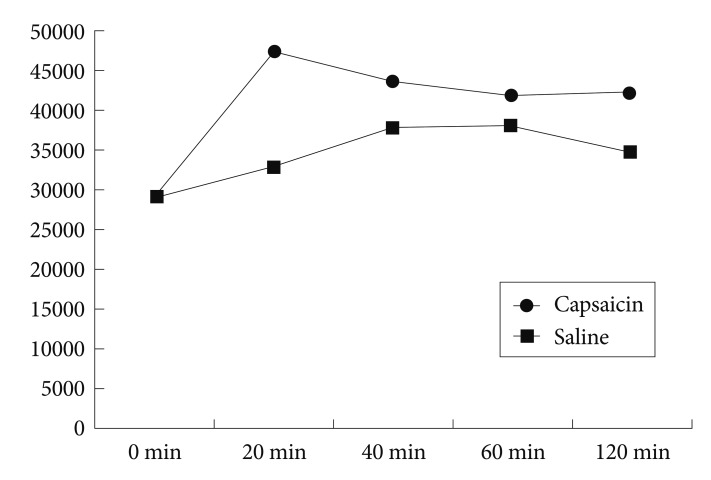
Capsaicin administration resulted in a significantly increased POMC mRNA level, compared to that in saline-treated rats at the 20-minute time point (t=-4.445, p=0.001). However, no significant group differences were observed at other times (t=-1.886, p=0.089; t= -0.973, p=0.353; t=-2.193, p=0.053 for 40, 60, and 120 minutes, respectively).
Conclusion
The analgesic effect of capsaicin might be associated with increased activity of the cerebral opioid system. This finding suggests that capsaicin acted for nociception and analgesia and could affect alcohol-intake behavior, which might further imply that a food culture could affect drinking behavior.
The mesolimbic dopamine pathway originating from the ventral tegmental area (VTA) and projecting to the nucleus accumbens (NA) is called the cerebral reward pathway and is closely related with the natural pleasures felt in daily life.1,2
This pathway can be activated by dependent substances. Therefore, the pleasure reward pathway is thought to be closely connected to addiction to substances such as alcohol, heroin, and cocaine. The cerebral opioid system plays an important role in mesolimbic dopamine pathway activity.
The concentration of dihydroxyphenylacetic acid (DOPAC) and dihydroxyphenylalanine (DOPA) increase following an intraventricular injection of opioid receptor agonist, whereas the incremental effect of DOPAC and DOPA by an agonist was blocked following an intraventricular injection of an opioid receptor antagonist.3 Spanagel et al.4 demonstrated these findings in a rodent model by reporting increased dopamine release in the NA when a ┬Ą-opioid receptor agonist was injected into the VTA, whereas decreased dopamine release was observed in the NA when a ┬Ą-opioid receptor antagonist was injected. It was recently reported that opioid neurons project to the NA and modulate activity of the mesolimbic dopamine pathway.5
Recent reports suggest an analgesic effect of capsaicin, despite being a noxious stimulant and main component of the hot flavor of red peppers when consumed by humans.6,7 These findings are supported by several previous studies suggesting that capsaicin is effective for relieving postherpetic or trigeminal neuralgia.6,8 Capsaicin is currently used for treating myalgia.9
The mechanism of the analgesic effect of capsaicin is thought to be the result of the neuropeptide depletion by long-term administration, as well as the release of ╬▓-endorphin, an endogenous peptide released from the brain to inhibit pain, and projected to the central nervous system through the spinal cord.10-13 The cell bodies of ╬▓-endorphin-producing neurons are predominantly located in the arcuate nucleus of the hypothalamus.10
Bach et al.10 reported increased cerebrospinal fluid (CSF) ╬▓-endorphin levels in the cerebellar cistern magna 45 minutes after a subcutaneous injection of capsaicin into white rats. Bach and Yaksh14 also reported an increased CSF ╬▓-endorphins in the cerebellar cistern magna at 30 minutes after an intrathecal injection of capsaicin into white rats, suggesting increased activity of the cerebral opioid system following capsaicin administration.
However, several reports have suggested that capsaicin activates the mesolimbic dopamine pathway through opioid activation as stated above. Some people prefer consuming pepper, even though they were previously abhorrent to red pepper due to the hot flavor.15,16 This phenomenon has been explained by pleasure seeking behavior due to the analgesic effect of ╬▓-endorphin increased by pain and opioid activation, thereby seeking hot flavor and promoting continuous ingestion.17,18
In this study, we investigated proopioimelanocortin (POMC) mRNA expression in the arcuate nucleus of Sprague-Dawley (SD) rats after administering capsaicin, hypothesizing that capsaicin induces activation of the mesolimbic dopamine reward pathway through activation of the central opioid system.
Ninety 4-week-old SD rats (Hyochang Science, Daegu, Korea) were housed in groups of five per cage with free access to water and food (Samyang Food, Seoul, Korea), and a diurnal 12 hour light cycle. They were allowed to adjust to the laboratory environment for 5 days. The animal care protocol used in this study was reviewed and approved by the Pusan National University Institutional Animal Care and Use Committee. Eighty SD rats were randomly divided into the saline- and capsaicin-injection groups. The remaining 10 rats were used for baseline data.
Capsaicin (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in propylene glycol (Sigma-Aldrich) and diluted with 0.9% normal saline. Capsaicin (0.01 mg/kg) was injected subcutaneously into rats in the capsaicin-injection group, whereas the same volume of vehicle diluted with saline without capsaicin was injected into the saline-injection group.
Ten SD rats in each group were sacrificed at 20, 40, 60, and 120 minutes after capsaicin administration under anesthesia, and their brains were removed. The remaining 10 rats were sacrificed at baseline (0 minutes).
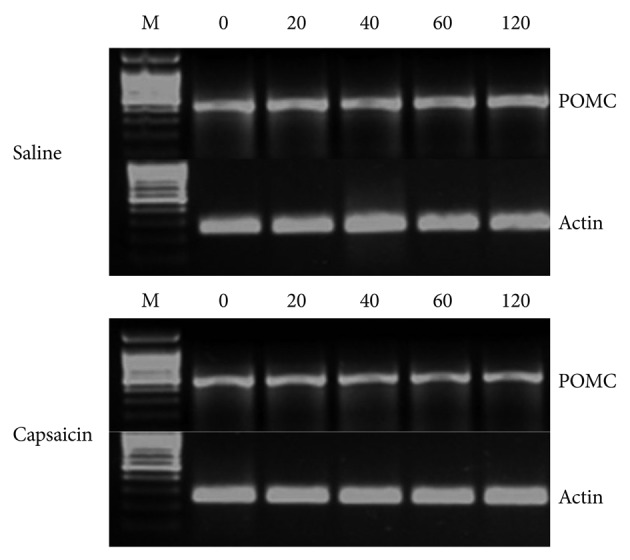
The POMC mRNA level in the arcuate nucleus was measured by RT-PCR as detailed below. The arcuate nucleus was excised from the brain after decapitation, and RNA was extracted using a Trizol reagent kit (Sigma Aldrich). The extracted total RNA was quantified with a spectrophotometer and prepared for RT-PCR. The POMC sequences were 436 bp fragments, 5'-GATTCTGCTACAGTCGCTC-3' for the forward primer and 5'-GAACTCTAGGGGAAAGGC-3' for the reverse primer (Bionics, Seoul, Korea). Single-standard POMC cDNA was amplified with an RT-PCR kit (Promega, Madison, WI, USA) in a 25 ┬ĄL reaction mixture containing 11 ┬ĄL of RNase-free water, AMV/Tfl 4 ┬ĄL of 5├Ś reaction buffer, 2 ┬ĄL dNTP mixture, 1 ┬ĄL forward primer, 1 ┬ĄL reverse primer, 0.8 ┬ĄL 25 mM MgSO4, 0.4 ┬ĄL AMV reverse transcriptase, 0.4 ┬ĄL Tfl DNA polymerase, and 1 ┬ĄL template RNA. The RT was performed at 45Ōäā for 45 minutes to maximize cDNA synthesis. The PCR was performed with a Perkin-Elmer GeneAmp PCR system 9600 (Waltham, MA, USA) with the following cycle parameters: one denaturation cycle for 2 minutes at 94Ōäā, 40 cycle of 30 seconds denaturation at 94Ōäā, 30 seconds annealing at 50Ōäā, and 1 minute extension at 72Ōäā. The final extension cycle for each reaction was 7 minutes at 72Ōäā.
The PCR products were fractionated on a 2% agarose gel (Sigma Aldrich) and stained with ethidium bromide. Bands intensities were measured with a densitometer (UVIpro Gel Documentation System; Topac, Cohasset, MA, USA).
Data are expressed as mean┬▒standard error, and the level of statistical significance was set at p<0.05. Analyses were performed using the Statistical Package for the Social Science (SPSS) 15.0 for Windows (Chicago, IL, USA). Differences between capsaicin- and saline-treated rats and between the baseline and other time points were tested by the independent t-test.
After a subcutaneous injection of capsaicin, SD rats had a significantly increased level of POMC mRNA in the arcuate nucleus of the hypothalamus at the 20-minute time point, compared to rats injected with saline, (t=-4.445, p=0.001). However, no significant differences between capsaicin- and saline-treated rats were observed at the 40, 60, and 120 minute time points (t=-1.886, p=0.089; t=-0.973, p=0.353; t=-2.193, p=0.053, respectively) (Figure 1 and 2).
Compared to baseline values, the level of POMC mRNA increased significantly at the 20, 40, 60, and 120-minute time points in capsaicin-treated rats (t=-24.719, p<0.001; t=-8.932, p<0.001; t=-4.387, p=0.006; t=-4.892, p=0.004, respectively). Similarly, POMC mRNA level increased significantly compared to baseline at the 40, 60, and 120-minute time points in saline-treated rats (t=-3.141, p=0.01; t=-3.141, p=0.01; t=-2.42, p=0.055, respectively). However, the level did not increase at the 20-minute time point (t=-1.185, p=0.287).
The purpose of this study was to assess the acute effects of capsaicin on POMC mRNA levels in the arcuate nucleus of SD rats. Capsaicin increased POMC gene expression more significantly than saline in the 20 minutes after a subcutaneous injection. These results demonstrate that activity in the cerebral opioid system increased following capsaicin administration.
POMC is a precursor of the potent opioid peptide ╬▓-endorphin as well as a number of other bioactive peptides including adrenocorticotropin and melanocyte-stimulating hormone (MSH). The major end products of POMC processing in the arcuate nucleus are ╬▓-endorphin and ╬▒-MSH.19 Capsaicin is an alkaloid derived from chili peppers and exerts its major pharmacological effects on the sensory nervous system, particularly on the C-fiber type primary afferent neurons. Previous studies have shown that CSF ╬▓-endorphin increases 45 minutes after a subcutaneous capsaicin injection10 and 30 minutes after an intrathecal injection.14 These results are similar to our results. However, the level of POMC mRNA in our study increased faster than that of CSF ╬▓-endorphin in other studies, which may have been due to the time required for the production and release of the peptides.
The saline injection alone also increased POMC mRNA level at the 40, 60, and 120-minute time points. A possible explanation for this saline-induced increase might be that pain and stress induced by laboratory procedures including saline injection caused such a change. For example, POMC mRNA level in the rat brain increases significantly in 1 hour or 3 days to 3 weeks under various stressful conditions such as electrical footshock, chronic arthritic pain, acute insulin-induced hypoglycemia, hypertonic saline loading, and exposure to cold.20 These results suggest that the capsaicin-induced increase in POMC mRNA level found in our study may have been underestimated in the statistical analysis. The POMC mRNA level of capsaicin-treated rats was significantly higher than that of saline-treated rats only at the 20-minute time point, although the levels were significantly higher than baseline at all time points.
In contrast, Panerai et al.21 reported that capsaicin induces a decrease in hypothalamic ╬▓-endorphin concentrations at 3, 5, and 7 days after administration, which return to normal values by 15 days. It was also reported that intraventricular infusion of capsaicin at 24 and 72 hours before collecting portal blood results in a 70% decrease in portal plasma ╬▓-endorphin concentrations compared to values in vehicle-treated rats.12 However, the exact mechanism of the capsaicin-induced decrease in ╬▓-endorphin level is unclear. Several hypotheses have been proposed. First, capsaicin has a neurotoxic action on POMC neurons, but there is no neuro-anatomical evidence for this effect. 22 Second, capsaicin may cause the release of substance p from the hypothalamus, which, in turn, may result in the depletion of hypothalamic ╬▓-endorphin.12 Third, POMC neurons could be autoregulated, as activation of opioid receptors decreases biosynthetic activity of POMC neurons, whereas an opiate receptor blockade causes an increase in the activity of these neurons.19 Fourth, a sustained release of ╬▓-endorphin could result in ┬Ą-opioid receptor phosphorylation and uncoupling of the receptor from effector systems, and, thus, desensitization.23
In our previous study we reported that alcohol intake was suppressed when rats were administered capsaicin compared with before capsaicin administration during the last 2 days.22 Neuropathic and chronic pain stimuli downregulate central ┬Ą-opioid and dopaminergic transmission; thus, neuropathic and chronic pain could decrease the reward effects of ┬Ą-opioid agonists (their abuse potential).23
In conclusion, we demonstrated that capsaicin increased POMC mRNA level at 20 minutes after a subcutaneous injection. The analgesic effect of capsaicin might be associated with increased activity of the cerebral opioid system. These findings suggest that capsaicin can affect alcohol-intake behavior, which might further imply that a food culture could affect drinking behavior.
Acknowledgments
This study was supported by Medical Research Institute Grant (2007-01), Pusan National University.
References
1. Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron 2002;36:229-240. PMID: 12383779.


2. Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev 1987;94:469-492. PMID: 3317472.



3. Manzanares J, Durham RA, Lookingland KJ, Moore KE. delta-Opioid receptor-mediated regulation of central dopaminergic neurons in the rat. Eur J Pharmacol 1993;249:107-112. PMID: 8282012.


4. Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A 1992;89:2046-2050. PMID: 1347943.



6. Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev 1991;43:143-201. PMID: 1852779.

7. Szolcsanyi J, Jancso-Gabor A, Joo F. Functional and fine structural characteristics of the sensory neuron blocking effect of capsaicin. Naunyn Schmiedebergs Arch Pharmacol 1975;287:157-169. PMID: 1143357.


8. Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev 1999;51:159-212. PMID: 10353985.

9. Robbins W. Clinical applications of capsaicinoids. Clin J Pain 2000;16(2 Suppl):S86-S89. PMID: 10870746.


10. Bach FW, Chaplan SR, Jang J, Yaksh TL. Cerebrospinal fluid beta-endorphin in models of hyperalgesia in the rat. Regul Pept 1995;59:79-86. PMID: 12506417.


11. Gamse R. Capsaicin and nociception in the rat and mouse. Possible role of substance P. Naunyn Schmiedebergs Arch Pharmacol 1982;320:205-216. PMID: 6182473.


12. Koenig JI, Meltzer HY, Gudelsky GA. Morphine or capsaicin administration alters the secretion of beta-endorphin into the hypophysial portal vasculature of the rat. Neuroendocrinology 1986;43:611-617. PMID: 2944026.


14. Bach FW, Yaksh TL. Release of beta-endorphin immunoreactivity into ventriculo-cisternal perfusate by lumbar intrathecal capsaicin in the rat. Brain Res 1995;701:192-200. PMID: 8925284.


15. Rozin P. Acquisition of food preferences and attitudes to food. Int J Obes 1980;4:356-363. PMID: 6998884.

16. Rozin P, Ebert L, Schull J. Some like it hot: a temporal analysis of hedonic responses to chili pepper. Appetite 1982;3:13-22. PMID: 7103463.


17. Rozin P. In: Green BG, Mason JR, Kare MRGetting to Like the Burn of Chili Pepper: Biological and Psy-chological and Cultural Factors. , editor. Chemical Irritation in the Nose and Throat. 1990,New York: Marcel Dekker.
18. Stern J, Stern M. Chili Nation. 1999,New York: Clarkson Potter.
19. Garcia de Yebenes E, Pelletier G. Opioid regulation of proopiomelanocortin (POMC) gene expression in the rat brain as studied by in situ hybridization. Neuropeptides 1993;25:91-94. PMID: 8413862.


20. Wu P, Childs GV. Changes in rat pituitary POMC mRNA after exposure to cold or a novel environment, detected by in situ hybridization. J Histochem Cytochem 1991;39:843-852. PMID: 1851778.


21. Panerai AE, Martini A, Locatelli V, Mantegazza P. Capsaicin decreases B-endorphin hypothalamic concentrations in the rat. Pharmacol Res Commun 1983;15:825-832. PMID: 6196795.


22. Kim SG. Effect of capsaicin on alcohol intake in C57BL/6 mice. J Korean Neuropsychiatr Assoc 2004;43:564-569.
23. Niikura K, Narita M, Butelman ER, Kreek MJ, Suzuki T. Neuropathic and chronic pain stimuli downregulate central mu-opioid and dopaminergic transmission. Trends Pharmacol Sci 2010;31:299-305. PMID: 20471111.