 |
 |
- Search
| Psychiatry Investig > Volume 9(1); 2012 > Article |
Abstract
Objective
Methods
Results
Conclusion
References
Figure 1

Figure 2

Figure 3
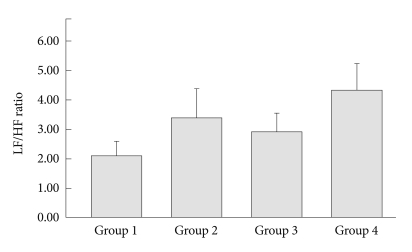
Figure 4
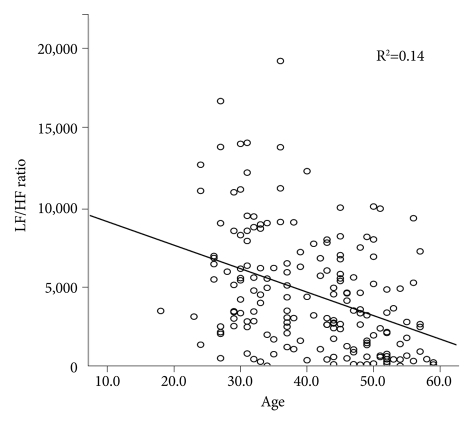
Figure 5
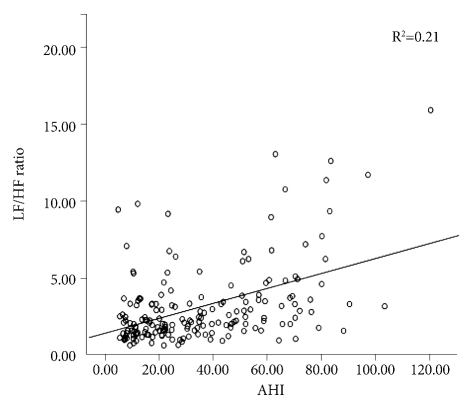
Table 1
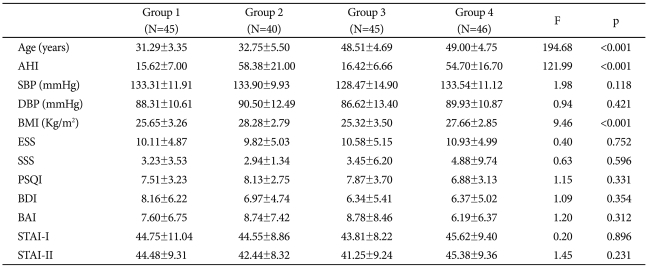
Group 1: young, mild to moderate OSAS group, Group2: young, severe OSAS group, Group3: middle-aged, mild to moderate OSAS group, Group 4: middle-aged, severe OSAS group. OSAS: obstructive sleep apnea syndrome, N: number, SD: standard deviation, AHI: Apnea-Hypopnea Index, SBP: systolic blood pressure, DBP: diastolic blood pressure, BMI: Body Mass Index, ESS: Epworth Sleepiness Scale, SSS: Stanford sleepiness scale, PSQI: Pittsburg Sleep Quality Index, BDI: Beck Depression Inventory, BAI: Beck Anxiety Inventory, STAI: State Trait Anxiety Inventory
Table 2

AHI: apnea-hypopnea index, SD: standard deviation, N: number, OSAS: obstructive sleep apnea syndrome, TIB: time in bed, SPT: sleep period time, TST: total sleep time, SL: sleep latency, SE: sleep efficiency, S1: stage 1 sleep, S2: stage 2 sleep, SWS: slow wave sleep, REM: rapid eye movement, ODI: oxygen desaturation index, LM: limb movement
Table 3
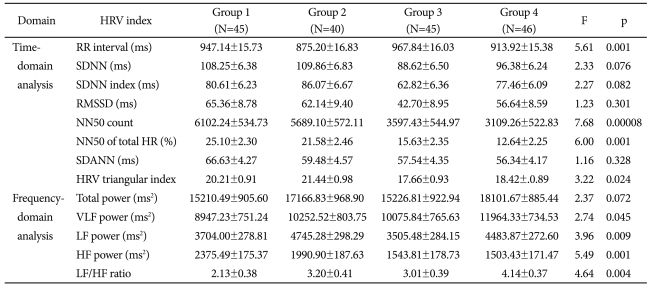
OSAS: obstructive sleep apnea syndrome, SDNN: standard deviation of all RR intervals, SDNN index: mean of the standard deviation of all RR intervals for all 5-min segments, RMSSD: square root of the mean of the sum of the squares of differences between adjacent RR intervals, NN50 count: the number of pairs of adjacent RR intervals differing by more than 50 ms in the entire analysis interval, NN50 of total HR (%): the NN50 count divided by the total number of all RR intervals, SDANN: the standard deviation of the averages of RR intervals in all 5-min segments, HRV triangular index: total number of RR intervals divided by maximum height of the histogram excluding boundaries, VLF: very low frequency, LF: low frequency, HF: high frequency, RR interval: the time between two consecutive R waves in the electrocardiogram, HR: Heart rate
Table 4

OSAS: obstructive sleep apnea syndrome, RR interval: the time between two consecutive R waves in the electrocardiogram, SDNN: standard deviation of all RR intervals, SDNN index: mean of the standard deviation of all RR intervals for all 5-min segments, RMSSD: square root of the mean of the sum of the squares of differences between adjacent RR intervals, NN50 count: the number of pairs of adjacent RR intervals differing by more than 50 ms in the entire analysis interval, NN50 of total HR (%): the NN50 count divided by the total number of all RR intervals, SDANN: the standard deviation of the averages of RR intervals in all 5-min segments, HRV triangular index: total number of RR intervals divided by maximum height of the histogram excluding boundaries, VLF: very low frequency, LF: low frequency, HF: high frequency
Table 5
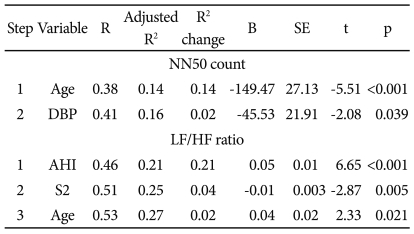
NN50 count: the number of pairs of adjacent RR intervals differing by more than 50 ms in the entire analysis interval, LF/HF ratio: Low frequency/high frequency ratio, OSAS: obstructive sleep apnea syndrome, SE: standard errors, DBP: diastolic blood pressure, AHI: Apnea-hyponea index, S2: stage 2 sleep






