fNIRS Assessment during an Emotional Stroop Task among Patients with Depression: Replication and Extension
Article information
Abstract
Objective
Accumulated evidence collected via functional near-infrared spectroscopy (fNIRS) has been reported with regard to mental disorders. A previous finding revealed that emotional words evoke left frontal cortex activity in patients with depression. The primary aim of the current study was to replicate this finding using an independent dataset and evaluate the brain region associated with the severity of depression using an emotional Stroop task.
Methods
Oxygenized and deoxygenized hemoglobin recording in the brain by fNIRS on 14 MDD patients and 20 normal controls.
Results
Hyperactivated oxygenized hemoglobin was observed in the left frontal cortex on exposure to unfavorable stimuli, but no significant difference was found among patients with depression compared with healthy controls on exposure to favorable stimuli. This result is consistent with previous findings. Moreover, an evoked wave associated with the left upper frontal cortex on favorable stimuli was inversely correlated with the severity of depression.
Conclusion
Our current work using fNIRS provides a potential clue regarding the location of depression symptom severity in the left upper frontal cortex. Future studies should verify our findings and expand them into a precise etiology of depression.
INTRODUCTION
Jöbsis [1] first reported a noninvasive infrared monitoring assessment of cerebral oxygen in the blood flow. Since then, many trials using near-infrared light on brain function have been performed [2,3]. Since a significant increment in oxygenated hemoglobin concentration ([oxy-Hb]) was first observed in the motor cortex upon finger movement [4], several groups have reported the utility of reflected or scattered light using near-infrared spectroscopy on brain activity during the same period [5-8]. In Japan, the world’s first commercialized functional near-infrared spectroscopy (fNIRS) method was developed in 1998. Hence, public medical insurance approved the device that uses this technology to investigate depression in 2014. Since then, fNIRS has been used in practical psychiatry and widely used across the country [9,10].
Although this first-of-its-kind device enabled investigations of biological distinctiveness with regard to psychiatric disorders within practical psychiatry, little is known about what fNIRS can capture in a living brain. One of the strongest criticisms of fNIRS is that it is assumed that the observed blood flow of the brain is largely affected by that of the skin [11,12]. Therefore, [oxy-Hb] captured via fNIRS cannot simply be considered to be the blood flow of the cortex. Our group recently published papers determining that fNIRS is a potential biomarker of the severity of the depressive state [13,14]. Associations between the integral value of [oxy-Hb] at the frontal cortex and the degree of depression scored on the Hamilton Depression Scale (HAMD) [15] as well as the Verbal Fluent Task (VFT) [16] were found, regardless of the type of psychiatric disorder. However, these findings still require validation based on neuroscientific background knowledge.
Many attempts have been made to assess task variety that can be used during a fNIRS assessment. Simply put, fNIRS assesses the change in hemoglobin during a task; therefore, it is crucial to use appropriate tasks for this assessment. Researchers have used visual [8], motor [17], language [18], and cognitive tasks [19] in individuals, whereas the VFT has been used for assessments covered by public medical insurance. Matsubara et al. [20] used an emotional Stroop task to assess the [oxy-Hb] and deoxygenated hemoglobin concentration ([deoxy-Hb]) in patients with major depressive disorder (MDD) and bipolar disorder (BD) who are in recovery. To elucidate the emotional change within both disorders, emotional words (e.g., “Smile,” “Love,” and “Peace” express happy feelings, whereas “War,” “Hostility,” and “Crime” express threat feelings) were shown during the Stroop task. Interestingly, their finding regarding the comparison between patients with MDD and healthy controls detected a significant change in the left middle frontal region during the threat task because this region is located above the dorsolateral prefrontal cortex (dlPFC), which is potentially influenced by fear stimuli. However, their study had a potential limitation: The patients were in remission, which affects their brain functions. We employed only the depression patients not in remission based on the Structured Clinical Interview for DSM-IV (SCID) assessment in the current analysis. In addition, their report did not calculate the correlation between depression severity as measured by the HAMD and the wave pattern. This value is of great interest because our recent work found that the integral value of [oxy-Hb] at the frontal lobe was correlated with the HAMD score. Therefore, the current study aimed to validate Matsubara et al.’s finding in another sample set and determine the specific brain region affected by the severity of depression during the emotional task employed by previous works combined with the use of fNIRS [13,14].
METHODS
Participants
Fourteen participants with MDD and twenty healthy controls (HCs) were included in our analysis. We selected participants with Major Depressive Disorder, Code 296.2 (Major depressive affective disorder, single episode)–296.3 (Major depressive affective disorder, recurrent episode), based on their SCID assessment which was conducted by the clinical trained psychologists. HCs were excluded from the subsequent analysis if they had histories of psychiatric disorders. Most of the participant with MDD was taking antidepressants regularly, although four patients without taking current antidepressant drug treatment. All participants underwent laboratory testing and physical examinations to exclude physical illness. If we found an abnormal physical condition, then the data of those participants were omitted from subsequent analyses. The demographic details of participants are shown in Table 1, and detailed content of SCID assessment is in Table 2.
The ethical committee at Osaka Medical College approved this study. All participants provided written informed consent after the study design was explained. This clinical investigation was also approved and registered in the National Registration System of Japan (UMIN000013214).
Psychological assessment
Trained clinical psychologists assessed the participants’ tests in a blind manner. Subsequent tests were evaluated within two days, including a fNIRS assessment, Wechsler Adult Intelligence Scale (WAIS-III) [21] to calculate the intelligence quotient, and clinical diagnosis based on the diagnostic Diagnostic and Statistical Manual of Mental Disorders (DSM), the Structured Clinical Interview for the DSM-IV [22,23]. The assessments of WAIS-III and SCID were not performed on healthy controls.
fNIRS
A 22-channel NIRS (ETG-4000; Hitachi Medical, Tokyo, Japan) was used to derive the [oxy-Hb] and [deoxy-Hb] values. We placed 18 channels over the frontal cortex and four channels over the temporal cortex (Figure 1 shows the location of the probes). Briefly, participants sat comfortably in a quiet, well-lit room. The task procedures were explained by a clinical laboratory technician, who subsequently monitored participant head movement during the procedure. To avoid the effect of motion artifacts, data showing clear evidence of head movement were omitted from analysis. Additional details are described in our previous reports [13,14].

Activation regional patterns during the threat task in depressive patients. At left lower frontal cortex (Channel 17), significant increment of response in MDD patients was observed compared to NCs during the threat task (p=0.00016). Hb: Hemoglobin, MDD: Major Depressive Disorder, NCs: Normal Controls.
Emotional stroop task
During the fNIRS assessment of the participants, an emotional Stroop task was conducted. This task was designed using Presentation® (Neurobehavioral Systems, Inc., Berkeley, CA, USA). Participants were asked to read a word written in kanji (two Chinese characters) and respond to the color of the words, while also ignoring the meaning of words that represent three emotions such as “Neutral,” “Happy,” and “Threat”. Participants performed 3 trials that lasted for 6 min. A trial consisted of 30 words per emotion for 60 sec and 30 words from the neutral category for 60 sec (Figure 2). Fifteen words from the neutral category were shown for 30 sec as a pre-task. Subsequently, 3 experimental block trials were displayed. The words appeared pseudo-randomly in red, green, blue, or yellow on a black background. Words with the same color were not repeated consecutively. Each word was displayed for 2 sec on a computer screen, and no emotional word was repeated. Participants were instructed to respond as quickly as possible by pressing 1 of 4 keys on a computer that corresponded to the color of the word. The design was adopted from Matsubara et al.’s previous work [20] under their guidance.
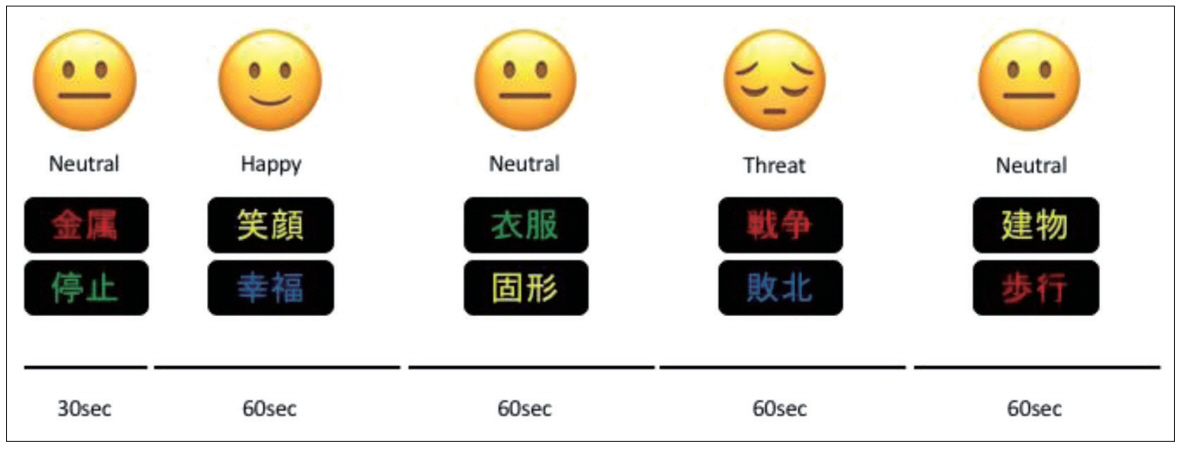
Emotional stroop task. At reading a word written in kanji (two Chinese characters) and respond to the color of the words by pushing the four colored keys. The meaning of kanji induces three emotions such as “Neutral,” “Happy,” and “Threat”. Trial design consisted of 30 words per emotions and neutral for 60 sec, respectively. Displayed task was designed using Presentation® software (Neurobehavioral Systems, Inc.).
Statistical analyses
Based on the previous statistical method used by Matsubara et al.’s previous work [20], the [oxy-Hb] and [deoxy-Hb] changes between the two groups (MDD and HCs) with regard to the emotional Stroop task were analyzed. We applied a false discovery rate (FDR) correction set at p<0.05, and Student’s t-test was used to detect the channel(s) with statistical significance (p<0.05). During the activation task, changes in the two parameters ([oxy-Hb] and [deoxy-Hb]) were examined after controlling for certain clinical information, including age of illness onset and duration of the disorder at the channel showing a significant difference based on Spearman’s correlation coefficient.
RESULTS
Replication of Matsubara’s study [20]
By evaluating another sample set featuring both patients with MDD and HCs, we assessed [oxy-Hb] in the frontal cortex and temporal lobes using a set of probes. During the threat task, a hyperactivated pattern was observed in the left lower prefrontal cortex (MDD: 0.041±0.063 mmol/L·mm, HCs: -0.027±0.052 mmol/L.mm, p=0.0016) (Figure 1). This finding is consistent with a previous report; however, a slight difference in height was observed in the left frontal cortex with regard to the activation pattern during the Threat task (which was displayed in the middle in the previous report, but lower in the current study). In addition, we did not observe a difference in the Happy task in our dataset, which also corroborates Matsubara’s report (Figure 3).

The comparison between Matsubara’s report. At the threat task, activation of left frontal region were observed in both datasets of MDD patients, while non-significant finding in any channels have been found at the happy task in both datasets. Hb: Hemoglobin, MDD: Major Depressive Disorder, NCs: Normal Controls.
HAMD assessment and Verbal fluent task performance
Because the use of fNIRS to examine patients with depression requires an assessment of the Verbal fluent task [24] based on the approval of Japanese public health insurance [10], we recorded the blood flow during the VFT on the same day by adding the emotional Stroop task. During the VFT, patients speak the number of words beginning with a specified letter (e.g., A, T, P, and so on). Participants are asked to speak as many words as they are able. During the executive function task, the frontal cortex is activated [25]. Our previous work revealed a significant correlation between the severity of depression and the integral value of [oxy-Hb] [13]. Therefore, the probe attached to the brain, which is a biomarker of the severity of depression during the emotional Stroop task, is of great interest. The data indicated channel 3 (between probes 3 and 4), which was located in the left upper frontal cortex. On that channel, [oxy-Hb] was hypoactivated in patients with MDD compared with HCs during the Happy task. This impaired response was directly related to the integral value of [oxy-Hb] during the VFT task. Adding to our previous finding, the blood flow at this position represents a biomarker of depression severity (Figure 4).

The location of CH3 (right upper figure) and inverse correction between CH3 activity and HAMD score. Left upper figure shows the inverse correlation between severity of depression and the integral value of [oxy-Hb] at the frontal lobe (N=42, F=5.94, p=0.02) in previous report. Current result of MDD patients (N=14) indicates significant correlation between the integral value of [oxy-Hb] at the frontal lobe and the value of CH3 at the happy task. Hb: Hemoglobin, MDD: Major Depressive Disorder, NCs: Normal Controls, CH: Channel, HAMD: Hamilton Depression Rating Scale.
DISCUSSION
The current work aimed to verify the previous finding by Matsubara et al. [20] and expand neuroscientific knowledge about the location in the brain that might be impaired with regard to emotional stimuli based on depression severity. Assessing the current independent dataset, a similar tendency for MDD was found. During the Threat task, a hyperactivated [oxy-Hb] pattern in a left frontal region (i.e., the dorsolateral prefrontal cortex, dlPFC) and non-significant difference during the Happy task was replicated (Figure 3). In addition, because of the interest in which location in the brain is impaired based on the severity of depression on exposure to the emotional stimuli, a correlation was found between the signal in the left upper frontal region (i.e., the dorsomedial prefrontal cortex, dmPFC) and an integral value on the VFT obtained by our previous report (Figure 4), while the association between HAMD assessment and the evoked pattern was not directly seen in the current sample. The proposed mechanism in the medial PFC is self-referential [26,27], which requires high blood flow even in a resting state [28,29]. The concept of the self-referential process was developed during the task that presented stimuli with thinking and self-related judgment. For instance, one study revealed hypoactivity in the cortical midline structure of patients with depression, showing pictures under two conditions: passive viewing or self-related judgment [30,31]. In patients with acute depression, negative pervasive thought, especially with regard to self-related matters, are often observed. In the current study, positive emotional stimuli were evoked by the integral value of the VFT in the dmPFC, which implies that the severity of depression is inversely associated with the gained value in the dmPFC. In other words, patients with more severe depression will likely show a decreased response in the dmPFC upon exposure to favorable stimuli. To our knowledge, this report is the first to use fNIRS and emotional stimuli to show an inverse response at the dmPFC.
A recent review with regard to the medial PFC in the patients with depression that summarized functional MRI research showed a different type of activation, including an elevated phasic pattern in the dorsal PFC and an elevated tonic pattern in the ventral mPFC [32]. Increased mPFC activation leads to biased attention, biased processing, and biased memory and rumination [33]. This negative cognitive bias is the central etiology of depression, although a detailed neurobiological mechanism has yet to be found. The current finding regarding the dmPFC using fNIRS and an emotional task has the potential to illuminate the neurological causes of depression. The status of depression might have affected the obtained fNIRS data. A recent analysis assessing pre- and post-treatment of depression showed a consistent wave pattern on the VFT [34], which leads the response in the frontal cortex be regarded as a trait marker. A previous report recruited patients in remission [20], whereas our sample was composed of individuals not in remission in terms of depression treatment.
We found consistent results on the emotional Stroop task, regardless of the stage of depression treatment, whereas other groups reported an NIRS wave that could have been influenced by anxiety and obsession-compulsion symptoms [35] or positive and negative automatic thought [36]. Apart from the assessment on MDD, there is a growing evidence correlated with the altered NIRS and different psychiatric disorders. For example, a series of NIRS assessment in schizophrenia or autism spectrum disorders indicated that prefrontal cortex activity is related to the social cognitive function [37], and Cognitive rehabilitation [38]. In a subsequent study, a larger, clinically homogenous MDD sample is needed to study the manifestation of fNIRS in depression.
Acknowledgements
We deeply thank Mrs. Toshie Ide for her technical assistance on fNIRS recording. In addition, we appreciate the clinical psychologists for their precise assessment on psychological status, intelligence quotient, and SCID. Finally, we always appreciate the patience of all of participants in the study.
This work was supported by JSPS KAKENHI, Grant Numbers 15K15433 and 16K10196 (PI: Tetsufumi Kanazawa).