Association between Obsessive-Compulsive Symptoms and Long-Term Cardiac Outcomes in Patients with Acute Coronary Syndrome: Effects of Depression Comorbidity and Treatment
Article information
Abstract
Objective
The role of obsessive-compulsive symptoms (OCS) in patients with acute coronary syndrome (ACS) is not well elucidated. This study investigated the association between OCS and the long-term prognosis of ACS in tandem with depression comorbidity and treatment.
Methods
A cross-sectional baseline study and a nested 24-week double-blind escitalopram-placebo controlled trial were carried out between May 2007 and March 2013, and then a 5–12-year follow-up for major adverse cardiac events (MACE) was conducted. A total of 1,152 patients with ACS were stratified by baseline depression comorbidity and treatment allocation into four groups: no depression (706 patients), depression and taking escitalopram (149 patients), depression and taking a placebo (151 patients), and depression and receiving medical care as usual (CAU; 146 patients). OCS were evaluated using the Symptom Checklist-90-Revised Obsessive-Compulsive symptom domain. During the follow-up, Kaplan-Meier event rates for MACE outcomes were calculated, and hazard ratios were estimated using Cox regression models after adjusting for a range of covariates.
Results
A higher OCS score at baseline was associated with a worse ACS prognosis after adjusting for relevant covariates and across MACE outcomes. This association varied according to the depression comorbidity. The association was significant in patients without depression and depressive patients receiving placebos and CAU, but not in depressive patients on escitalopram.
Conclusion
Evaluating OCS and depression is recommended during the early phase of ACS. Treatment for OCS may improve the long-term cardiac outcomes of patients with ACS.
INTRODUCTION
Acute coronary syndrome (ACS) is a life-threatening disease and a significant disease burden associated with high morbidity and mortality [1]. A risk-factor evaluation is important for ACS patients, and several psychosocial variables have been considered prognostic predictors of ACS. Depression has been most extensively investigated, but anxiety, social isolation, and stress have also received research [2].
Of the psychosocial factors, obsessive-compulsive symptoms (OCS) or disorder (OCD) belonged to the “anxiety disorder” category before the introduction of the Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (DSM-5) [3]. Therefore, most previous studies on ACS have focused on anxiety rather than on OCS separately. Moreover, studies on the role of OCS on ACS prognosis are scarce and have presented inconclusive findings. Two cross-sectional studies reported that OCS are more common in patients with ACS than those without [4,5]. A cohort study evaluated anxiety disorder as an ACS prognostic factor and reported that OCD was not associated with readmission and/or cardiac mortality at 12 months after the ACS event, but was significantly associated with them at 5 years. However, fewer than 20 OCD patients were observed [6,7]. No other studies have evaluated the direct association between OCS and the ACS prognosis. Previous studies have many limitations, such as a small sample size, short follow-up period, or methodological problems.
Depression may be an important factor that impacts the association between OCS and cardiac outcomes in patients with ACS, as depression is a common comorbidity of OCD [8]. A comorbid depressive feature in patients with OCD is accepted as a poor-prognosis factor [9], and antidepressants, particularly selective serotonin reuptake inhibitors (SSRIs), are effective for treating both OCS and depression [10]. However, no study has considered depression comorbidity and treatment to investigate this issue. To resolve these unanswered questions, we investigated the effects of OCS on the long-term cardiac outcomes of ACS, as well as further evaluated this association according to the status of depression comorbidity and antidepressant treatment.
METHODS
Study overview and participants
The present analyses were performed using data from a larger naturalistic study of patients with ACS—the Korean DEPression in ACS (K-DEPACS) study, which also included a nested randomized clinical trial for patients with depression and ACS—the Escitalopram for DEPression in ACS (Es-DEPACS) study. The original design and main findings of the K-DEPACS and EsDEPACS studies have been published [11,12], and eligibility criteria are described in the online Supplementary Material. Written informed consent was collected for both studies, which were approved by the Chonnam National University Hospital Institutional Review Board (IRB No. 06-050). The outline and participant-recruitment process for the present analysis are presented in Figure 1.
K-DEPACS baseline evaluations
Participants were recruited from patients who were hospitalized due to ACS (n=4,809) at the Department of Cardiology, Chonnam National University Hospital, Gwangju, South Korea from 2006 to 2012. This department was nominated by the Korean Circulation Society to serve as the central coordinating center for the Korea Acute Myocardial Infarction Registry (KAMIR) because of the large number of ACS patients seen here and the high quality of data acquisition and management here [13]. Patients were treated by the study cardiologists based on international guidelines for managing ACS [14]. Eligible participants (n=1,152) were examined for baseline evaluations as inpatients within 2 weeks post-ACS, with a mean of 6.3±2.4 [standard deviation (SD)] days. OCS were evaluated using the Symptom Checklist-90-Revised (SCL-90-R) [15]. This is a self-administered 90-item questionnaire consisting of multiple-choice questions with a 5-point (1–5) scale of distress, ranging from “not-at-all” to “extremely distressed.” The Obsessive-Compulsive symptom dimension of the SCL-90-R consists of 10 of the total 90 items. The SCL-90-R is used widely in clinical situations and in research because of its appropriate design for use in individuals with medical conditions [15] including cardiac disorders [16,17]. Major or minor depressive disorder was diagnosed by psychiatrists using the Mini-International Neuropsychiatric Interview, a structured diagnostic psychiatric interview for disorders in the DSM-IV [18]. According to these criteria, patients are diagnosed with major depressive disorder if they have at least one core symptom (i.e., depressed mood or loss of interest) and at least four other symptoms of depression. A diagnosis of minor depressive disorder is made if patients have at least one core symptom and at least two others, but fewer than five symptoms in total.
Baseline characteristics that could potentially affect cardiac outcomes were collected [19]. Demographic data on age, gender, education, marital status, living alone, accommodation, and employment status were obtained. The self-completed Beck Depression Inventory (BDI) [20], and previous and family histories of depression were recorded to evaluate depressive symptoms. The following cardiovascular risk factors were ascertained: diagnosed hypertension and diabetes mellitus, hypercholesterolemia by fasting serum total cholesterol level (>200 mg/dL), obesity by the body mass index, reported current smoking status, and previous and family histories of ACS. Cardiac severity status was identified according to ACS diagnosis [myocardial infarction (MI) or unstable angina], Killip classification [21], left ventricular ejection fraction, and serum levels of troponin I and creatine kinase-MB.
The nested EsDEPACS study
Of the 446 individuals with a baseline diagnosis of a depressive disorder, 300 agreed to be randomized into the EsDEPACS study (ClinicalTrial.gov registry number: NCT00419471), a 24-week, double-blind, escitalopram (n=149) or placebo (n=151) controlled trial. The first patient was enrolled in May 2007 and the last patient completed the follow-up evaluation in March 2013. Flexible doses of escitalopram (5, 10, 15, or 20 mg) or a matched placebo were administered and determined according to the investigators’ clinical decision, considering the patients’ response and tolerance. Mean doses at the last visit were 7.6±3.7 mg for the escitalopram group and 8.5±3.9 mg for the placebo group. Examinations were scheduled at baseline, and at weeks 4, 8, 12, 16, 20, and 24 thereafter. As previously reported, the main results of this trial demonstrated the superiority of the escitalopram treatment for alleviating depressive symptoms [11]. The remaining 146 patients who declined participation (n=123) or were ineligible (n=23; 12 for taking disallowed drugs, 6 with a history of neuropsychiatric illness, and 5 for participating in other drug trials) for participation in the trial received medical care as usual (CAU) for ACS. Higher participation rates were noted in those with more severe depression (i.e., major vs. minor depressive disorder). Of patients receiving escitalopram and a placebo, 57.0% (85/149) and 55.6% (84/151) were diagnosed with a major depressive disorder, respectively, compared to 22.6% (33/146) of patients who received CAU.
Long-term cardiac outcomes
All participants were approached for follow-up evaluations of cardiac outcomes in 2017, 5–12 years after the index ACS, or until they died. The median follow-up duration was 8.4 years, and the mean was 8.7±1.5 years. Comprehensive evaluations for cardiac outcomes were possible for this study because KAMIR manages and records detailed electronic data on hospital admissions, deaths, recurrent MI, and percutaneous coronary intervention (PCI). The primary endpoint was a major adverse cardiac event (MACE), which was a composite of all-cause mortality, MI, and PCI. Secondary endpoints were all-cause mortality, cardiac death (defined as sudden death for which no other explanation was available; death from arrhythmia, or after MI or heart failure; or death caused by heart surgery or endocarditis), MI, and PCI. An independent endpoint committee composed of study cardiologists adjudicated all potential events and was blinded to the participants’ psychiatric comorbidities and the treatment randomization status.
Statistical analyses
As there have been no validated cutoffs published to categorize the severity of OCS, scores on the Obsessive-Compulsive symptom dimension of the SCL-90-R were dichotomized at the median value into less- and more-severe-OCS groups. Although this classification was arbitrary, it has been used in previous studies [22]. Baseline demographic and clinical characteristics were compared between the less- and more-severe-OCS groups using t-tests or χ2 tests, as appropriate. Characteristics significantly associated with OCS (p<0.05) and other variables with potential effects on MACE were used as covariates in further adjusted analyses [19]. Kaplan-Meier models were used to compare the cumulative proportion of participants experiencing a composite and individual MACE (defined according to the date of the first event for each patient) between those with less- or more-severe OCS. Cox proportional hazards models were used to compare time to the first composite and individual MACE after adjusting for the potential covariates described above between the two groups. According to the depression comorbidity and treatment status at baseline, the patients were divided into four groups: no depression (n=706), depression and taking escitalopram (n=149), depression and taking a placebo (n=151), and depression and receiving CAU (n=146). To evaluate the effects of depression comorbidity and treatment status on the associations between OCS and a composite and individual MACE, the same Cox proportional hazards models were used for these four groups separately, and the interaction terms were calculated after adjusting for all potential covariates. Additional sensitivity analyses were conducted using severity of OCS as a continuous exposure variable to re-examine its effect beyond the binary categorical approach. All statistical tests were two-sided with a significance level of 0.05. Statistical analyses were carried out using SPSS software (ver. 21.0; IBM Corporation, Armonk, NY, USA).
RESULTS
Baseline demographic and clinical characteristics by severity of OCS
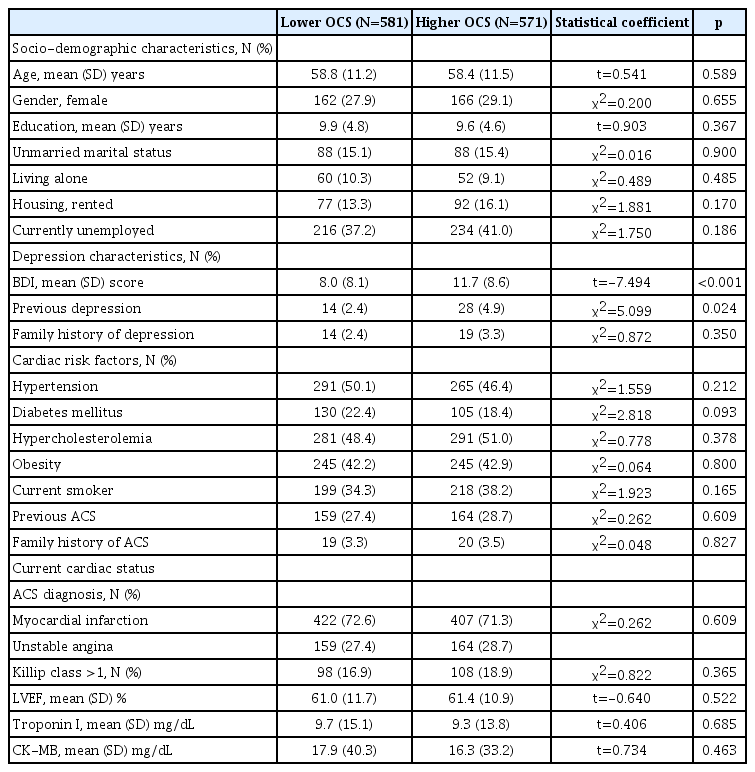
Median and mean values of the OCS dimension based on the SCL-90-R were 13.0 (interquartile range, 11.0–15.0) and 13.8±4.1, respectively. The baseline characteristics between patients with less- and more-severe OCS are compared in Table 1. More-severe OCS were significantly associated with a higher BDI score and a history of depression. These characteristics were included as covariates in subsequent analyses. In addition, age, education, all cardiac risk factors, and current cardiac status variables were also considered covariates because they have been associated with cardiac outcomes in previous studies [19,23].
Effects of OCS on the occurrence of a MACE
The primary endpoint (composite MACE) occurred in 446 (38.7%) participants. Considering secondary endpoints, all-cause mortality occurred in 211 (18.3%), cardiac death in 111 (9.6%), MI in 110 (9.5%), and PCI in 162 (14.1%) participants. Figure 2 illustrates the cumulative risk of a composite MACE in subjects with less- and more-severe OCS. A significant difference was observed—composite MACE incidences were 31.3% (182/581) and 46.2% (264/571) in the less- and more-severe-OCS groups (long-rank p<0.001), respectively. Figure 3 illustrates the cumulative risk of individual MACE components in the two groups. Significant differences were observed in the incidences of all-cause mortality (16.0% and 20.7% in the less- and more-severe-OCS groups, respectively; log-rank p=0.035), MI (6.9% and 12.3% in the less- and more-severe-OCS groups, respectively; log-rank p=0.001), and PCI (10.7% and 17.5% in the less- and more-severe-OCS groups, respectively; log-rank p=0.001), but not in the incidence of cardiac death. The effects of more-severe OCS at baseline on MACE outcomes after adjustment are summarized as hazards ratios with 95% confidence intervals in parentheses in the “all participants” rows of Table 2. The strength of the associations decreased, but remained significant for composite MACE, allcause mortality, MI, and PCI outcomes after adjusting for age, education, BDI score, history of depression, hypertension, diabetes, dyslipidemia, obesity, smoking, previous and family history of ACS, ACS diagnosis, Killip class, left ventricular ejection fraction, and serum levels of troponin I and creatine kinase-MB at baseline.
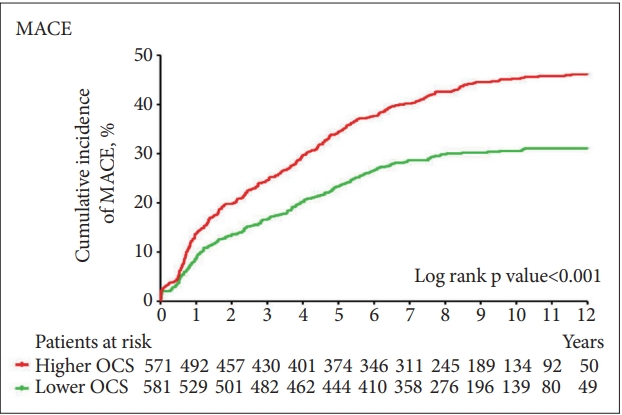
Cumulative incidence of composite MACEs. MACE: major adverse cardiac events, OCS: obsessive-compulsive symptoms.

Cumulative incidence of the individual major adverse cardiac event components of allcause mortality, cardiac death, MI, and PCI. MI: myocardial infarction, PCI: percutaneous coronary intervention, OCS: obsessive-compulsive symptoms.
Effects according to depression comorbidity and treatment status
The effects of OCS on the incidences of MACE according to depression comorbidity and treatment status are summarized in Table 2. More-severe OCS at baseline in participants without depression were significantly associated with a composite MACE but were not associated with any individual MACE outcome. These significant associations were not found with any MACE outcome in depressed patients taking escitalopam. Significant associations were detected with composite MACE and MI outcomes in depressed patients taking a placebo. In depressive patients receiving CAU, there were significant associations with a composite MACE, and MI and PCI outcomes. All of these associations were similar before and after adjustment. The results of additional sensitivity analyses using the scores based on the Obsessive-Compulsive symptom dimension of the SCL-90-R as continuous variables are summarized in Supplementary Table 1 (in the online-only Data Supplement). The strengths of the associations did not change substantially but the p-values were slightly higher compared to those from the analysis using the scale as a binary variable.
DISCUSSION
The main findings were that more-severe OCS at the early phase of ACS was associated with worse long-term cardiac outcomes, but this association varied according to the depression comorbidity and treatment status—it was significant in patients without depression and in depressed patients taking a placebo or receiving CAU, but not in depressed patients taking escitalopram. These findings were robust after adjusting for the relevant covariates and across MACE outcomes.
Several mechanisms are plausible for the observed associations between more-severe OCS and worse prognosis of ACS. First, patients with OCD have relatively low serum adiponectin and high serum resistin levels compared to those of normal controls [24,25]. Adiponectin and resistin are related to metabolic disturbances, including cholesterol levels and glucose tolerance [25]. Therefore, ACS patients with more-severe OCS are more liable to have metabolic syndrome in a long-term follow-up. Similarly, OCD patients in the general population have higher rates of atherosclerosis than those without OCD [26]. Moreover, OCD is associated with an increased risk of metabolic and cardiovascular complications after the mean 22-year follow-up [27]. In the present study, cardiac risk factors, including diabetes mellitus and hypercholesterolemia, did not differ between the less- and more-severe-OCS groups at baseline (Table 1). However, OCS may have contributed to metabolic disturbances over the 5–12-year follow-up period, although follow-up examinations were not carried out. Second, patients with OCD have persistent anxiety and therefore have an exhausted autonomic nervous system [28]. In a functional brain magnetic resonance imaging study, activities increased in the limbic and paralimbic areas, indicating that the dorsolateral prefrontal cortex was hyperactivated in OCD patients [29]. A meta-analysis reported that OCD results in less total sleep time and a higher amount of awake time than in people without OCD. This result is similar to how obstructive sleep apneahypopnea affects the autonomic nervous system by hypo-oxygenation, which is a well-known risk factor for ACS [30]. Third, individuals with OCD have a core cognitive deficit called “low tolerance for uncertainty,” which gives rise to compulsive behavior [31]. Therefore, ACS patients with more-severe OCS are likely to have concerns about uncertainty that would worsen their heart problems. A controlled study using a gambling task revealed that OCD patients exhibited neuropsychiatric deficits. It is suspected that compulsions represent immediate rewards; thus, OCD patients are less sensitive to the future consequences of their choices [32]. This psychological impairment in OCD patients seems to make them adhere less frequently to medical treatment, which leads to a recurrence of ACS. Moritz reported lower scores for social and emotional quality of life (QOL) variables, as physical QOL in OCD is compromised relative to healthy subjects both before and after biopsychosocial OCD treatment [33].
Notably, OCS did not affect ACS prognosis as much when patients received escitalopram treatment. This finding could be explained several ways. First, the antidepressive effect of escitalopram should be considered. We have reported that escitalopram was effective for treating depression in patients with ACS and improved long-term cardiac outcomes [11]. In this study, OCS were significantly correlated with depressive symptoms evaluated using the BDI (Table 1); therefore, OCS may have improved along with the improvement in depression. However, the associations between OCS and cardiac outcomes remained significant after adjusting for depressive symptoms. Second, escitalopram is also used for treating OCD and OCS [34]. Therefore, the anti-OCS effect of escitalopram itself may have mitigated the negative consequences. Similarly, a Swedish nationwide cohort study reported that patients with OCD taking SSRIs had significantly lower risks of metabolic and cardiovascular complications compared with those who were not taking an SSRI [27]. High SSRI doses are needed conventionally to treat OCD [34]. Although lower doses (mean, 7.6 mg) were used in the present study, OCS rather than OCD were evaluated. Third, escitalopram may normalize metabolic and autonomic disturbances, which have adverse effects on cardiac outcomes [35,36].
Several issues should be considered before drawing conclusions. First, OCS rather than the diagnosis of OCD were evaluated and treated as an exposure variable. OCS are less formal and less accurate than OCD for categorizing the disease state, thus this categorization may have led to overestimates of the strength of the association. However, the numbers of OCD patients in previous studies were very low [6,7]; therefore, it would be difficult to draw conclusions due to limited statistical power. In addition, OCS may reflect general distress more than OCD per se [37]. In the present study, the association was confirmed using the score on the Obsessive-Compulsive symptom dimension of the SCL-90-R as a binary and continuous variable. Second, OCS were evaluated using the Obsessive-Compulsive symptom dimension of the SCL-90-R. The Yale-Brown Obsessive-Compulsive Scale (YBOCS) is the gold-standard method for evaluating OCS [38]. However, there are studies showing that the Obsessive-Compulsive symptom dimension of the SCL-90-R has strong internal consistency [15,37], and a moderate to high correlation with the YBOCS [39]. Moreover, the SCL-90-R is a useful and convenient tool to screen psychiatric disease in various settings, including in physical disorder clinics worldwide [40]. Third, recruitment was carried out at a single site, which may limit the generalizability of the present findings. However, a single center study has potential strengths in terms of consistency in evaluation and treatment. Other considerations are that no data were available on patients who refused to participate in the recruitment process of the K-DEPACS baseline participants; therefore, the representativeness of the sample cannot be confirmed. Additionally, the CAU group was not randomly assigned but was formed by patients with depression who refused or were ineligible to take part in the clinical trial; and no attempt was made to investigate the effect of treatment (or a placebo) for depression on OCS during the follow-up period.
Nevertheless, our study had several strengths. The follow-up for cardiac outcomes was comprehensive, long, and complete. The study design was unique in that a prospective observational study and a randomized placebo controlled interventional study were conducted. Participants were recruited at baseline consecutively from all eligible patients with recent ACS, which increased sample homogeneity. All measurements, including those based on the SCL-90-R for psychiatric and cardiovascular characteristics, were well validated. A range of covariates was considered in the analyses.
In summary, OCS had adverse effects on ACS prognosis, but possible beneficial modifying effects of treatment were found. These findings have several important implications. From a clinical perspective, evaluations of OCS along with depression are recommended for patients who recently developed ACS. The SCL-90-R questionnaire is simple and easy for ACS patients to complete even at the early hospitalization stage. This procedure could make it more likely for further appropriate treatment to improve long-term cardiac outcomes. Studies about other antidepressants or non-pharmacological psychiatric treatments, such as cognitive behavioral therapy for the more-severe-OCS group, are recommended to confirm the present findings. Additional time and medical costs may be involved besides the usual cardiologic practice; therefore, future studies need to investigate both the efficacy and cost-effectiveness of any intervention.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.30773/pi.2019.0259.
Acknowledgements
This study was funded by a National Research Foundation of Korea grant (NRF-2015M3C7A1028899) and was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (grant NRF-2016R 1A2A2A05919518).
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Jae-Min Kim, Jin-Sang Yoon, Young-Keun Ahn. Data curation: Lee-Hee Joon, Hee-Joo Kang. Formal analysis: Sung-Wan Kim, Hee-Joon Lee, Young-Joon Hong. Funding acquisition: Il-Seon Shin. Investigation: Lee-Hee Joon, Ju-Wan Kim, Jae-Min Kim. Methodology: Hee-Joo Kang, Myung-Ho Jeong. Project administration: Jae-Min Kim. Resources: Young-Joon Hong, Myung-Ho Jeong. Software: Hee-Joo Kang, Young-Keun Ahn. Supervision: Jin-Sang Yoon. Validation: Ju-Wan Kim. Visualization: Hee-Joon Lee. Writing—original draft: Hee-Joon Lee, Jae-Min Kim. Writing—review & editing: Hee-Joon Lee, Jin-Sang Yoon, Jae-Min Kim.