Association of Alcohol Intake and Fracture Risk in Elderly Varied by Affected Bones: A Nationwide Longitudinal Study
Article information
Abstract
Objective
Previous studies investigating association of alcohol intake and fracture risk in elderly yielded conflicting results. We first examined the association between alcohol intake and total fracture risk in elderly subjects and further analyzed whether the association varied by fracture locations.
Methods
This is a nationwide population-based cohort study which included all people aged 66 (n=1,431,539) receiving the National Screening Program during 2009–2014. Time-to-event were defined as duration from study recruitment, the day they received health screening, to the occurrence of fracture.
Results
Total fracture was significantly lower in mild drinkers [adjusted hazard ratio (aHR)=0.952; 95% confidence interval (95% CI) =0.931–0.973] and higher in heavy drinkers (aHR=1.246; 95% CI=1.201–1.294) than non-drinkers. Risk pattern of alcohol consumption and fracture differed according to affected bones. Similar J-shaped trends were observed for vertebra fractures, but risk of limb fracture showed a linear relationship with alcohol intake. For hip fracture, risk decrement was more pronounced in mild and moderate drinkers, and significant increment was noted only in very severe drinkers [≥60 g/day; (aHR)=1.446; 1.162–1.801].
Conclusion
Light to moderate drinking generally lowered risk of fractures, but association between alcohol and fracture risk varied depending on the affected bone lesions.
INTRODUCTION
Fracture in elderly is a common and an important public health issue which contributes to high burden to healthcare services [1]. With population of elderly people growing, incidence of economic burden from fracture is also increasing worldwide. South Korea (hereafter “Korea”) is one of the fastest aging countries in the world, and its aging index is estimated to increase to 213.8% by 2030 [2]. Accordingly, economic burden of fracture in Koreans older than 65 years has already increased from US $88.8 million in 2007 to US $149.3 million in 2011 [3].
Excessive alcohol intake is an important risk factor of fracture [4]. Numerous cohort studies acknowledged that mild to moderate drinking does not increase, in fact decreases, risk of fracture, whereas heavy consumption is associated with greater fracture incidence [5,6]. A meta-analysis consisted of more than 3,700,000 participants also showed that relationship between alcohol consumption and hip fracture is non-linear with the light alcohol drinkers having the lowest risk [7]. However, studies also demonstrated that the risk factor of fracture varies with age [8,9]. Likewise, literature on the association of alcohol intake and fracture risk in elderly population is more diverse and complicated. In the Framingham study, moderate and heavy drinking were associated with a substantial risk of fracture in those aged less than 65 years, but there was only a marginal and non-significant increased risk in those aged 65 years or more [10]. In elderly group, the impact of alcohol in fracture incidence also differed depending on the affected bones. Greater alcohol intake was not associated with greater risk for non-spine fractures [11], whereas the risk increment was more evident for vertebral fractures [12]. In addition, a prospective study showed that alcohol intake had a U-shaped relationship with risk of hip fracture [13].
Despite contradictory association between alcohol intake and incidence of fracture in the elderly, all well sized cohort studies included subjects from various age groups [6,14]. In studies conducted in elderly population only, none of previous researches contained more than 150,000 subjects [15-17]. Moreover, all studies investigated impact of alcohol in limited number of bones, and none investigated whether the fracture risk differed depending on the skeletal lesions. To fill in this gap, we first aimed to examine the association of alcohol intake and risk of all fractures in elderly subjects using a large nationwide study from health insurance claims data. We further analyzed whether the association varied by fracture location.
METHODS
Data source
The Korean National Health Insurance Service (KNHIS) (http://nhis.or.kr/static/html/wbd/g/a/wbdga0101.html) is a mandatory public health insurance system, and it provides universal coverage to all residents Korea. All Koreans who are 40 or older were required, by KNHIS, to receive a compulsory health screening test every two years. The National Health Insurance Service-Health Screening Cohort (NHIS-HEALS) is a cohort who participated in this health screening programs. An additional health screening named National Screening Program for Transitional Ages (NSPTA) was initiated in 2007 for those aged 40 and 66 because they are regarded as transition to middle age and elderly respectively [18]. All these data are systematically stored and organized by National Health Information Database, which consists of healthcare data including health screening data, sociodemographic variables, and mortality for the whole Korean population https://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do. Numerous epidemiological studies using this database has been published, and its detail description can be found elsewhere [2,19,20].
Study population
We first included all subjects aged 66 who participated in the NSPTA program during 2009–2014 (n=1,555,103). Thereafter, 96,847 participants having either incomplete or missing data were excluded. A one-year lag was utilized, so those who developed fracture or died within one from the health screening day were excluded (n=26,717). Finally, a total of 1,431,539 participants were included in our study (Figure 1), and their mean duration of follow-up was 3.52±1.76 (maximum of 7) years. Information regarding health-related lifestyle, medical history, basic physical data (including body mass index and blood pressure), and clinical tests results are included in the questionnaire obtained during the mandatory health screening. This study was approved by the Institutional Review Board of Yeouido St. Mary’s Hospital, Seoul, Korea (No. SC19ZESI0124). Consent from individual subjects were not needed because the study used publicly open, anonymous data.
Exposure variable - alcohol consumption
Frequency and amount of alcohol consumption was based on questionnaire reports. All participants first responded to a close-ended question regarding frequency of alcohol consumption: “In average, how often do you drink alcohol per week?” They were instructed to choose a specific number ranging from 0 (none)–7 (7 days/week). In terms of amount of alcohol consumption, participants were then asked an open-ended question: “When you consume alcohol, how many drinks do you usually consume per day?” They were instructed to count amount of alcohol consumption to “drinks/day” regardless of alcohol type with a following detailed description: “a can of beer (355 cc) is equivalent to 1.6 drinks.”
Based on these reports, we quantified their alcohol intake to grams/day. The interpretation of a standard drink can vary from country to country, and we used the most conservative definition and calculated 1 standard drink to 8 grams of ethanol [21]. Thereafter, we calculated participants daily alcohol intake [(drinks/day×days/week×8 g/drinks)/7 days], and the participants were divided in to four groups: not drinkers (none), mild (<15 g/day), moderate (<30 g/day), and heavy drinkers (≥30 g/day).
Outcome variable-fractures
Participants having ICD-10 codes for vertebral fracture (S22.0, S22.1, S32.0, M48.4, and M48.5) and visited hospital more than twice due to same codes were defined as having vertebral fracture. Likewise, those having ICD-10 codes and visited hospital more than twice with fracture of upper arm (S42.0, S42.2, and S42.3), forearm (S52.5 and S52.6), or lower leg (S82.3, S82.5, and S82.6) were classified as having a limb fracture. Almost all elderly with hip fracture require either surgical treatment or supportive care via hospital admission. Thus, hip fracture was defined as having ICD-10 codes of and hospitalized to a hospital due to hip fracture (S72.0 and S72.1). Finally, total fracture included fractures of vertebra, limbs, hip, and others not listed above (i.e. S02.X for skull fracture, S12. X for neck fracture, S62.X for hand, S92.X for foot).
Statistical analysis
Difference between the 4 groups in baseline demographic and clinical characteristics were compared using analysis of variance (ANOVA) for continuous variables and Chi-squared test for categorical variables. The cohorts were followed from the day they received the health screening, to the occurrence of death, or the last follow-up day (December 31, 2016), whichever came first. Time-to-event were defined as the duration from study recruitment, the day they received health screening, to the occurrence of fracture. We performed Cox proportional-hazard regression, with none drinkers as reference category, to evaluate risk of total fracture. In addition, Cox proportional-hazard regression for fracture of vertebral, hip, and limbs were conducted to assess whether the association between alcohol consumption and fracture risk differed depending on the affected or fracture lesions. The cox proportional-hazard model was adjusted for potential confounding variables known to predict risk of fracture, which included gender, income, diabetes, hypertension, hyperlipidemia, smoking, physical exercise body mass index, and fracture history [1,22,23]. For all statistical analysis, we used SAS version 9.3 (SAS Institute, Cary, North Carolina, USA) with p-values<0.05 considered significant.
RESULTS
Participant characteristics
Among 1,431,539 participants, the number of subjects for none, mild, moderate, and heavy drinking groups were 1,027,575 (71.8%), 247,669 (17.3%), 88,643 (6.2%), and 67,652 (4.7%) respectively. The four groups did not differ in age because they were all enrolled at age of 66. Frequency and amount of alcohol intake increased sequentially from none to heave drinking group illustrating that the participants were properly allocated based on their drinking habits and intensity. Male ratio was lowest in none group and highest in heavy drinking group. Diabetes, hypertension, weight, height, triglyceride level, smokers, waist circumference, and blood pressure were higher in moderate ~ heavy drinking groups than none ~ low drinking groups. In contrast, rate of hyperlipidemia decreased sequentially from none to heave drinking group. History of fall was also more prevalent in none drinking group than other drinking groups. Lastly, a reversed-U shape was observed between physical activity and drinking groups (Table 1).
Group differences in fracture risk
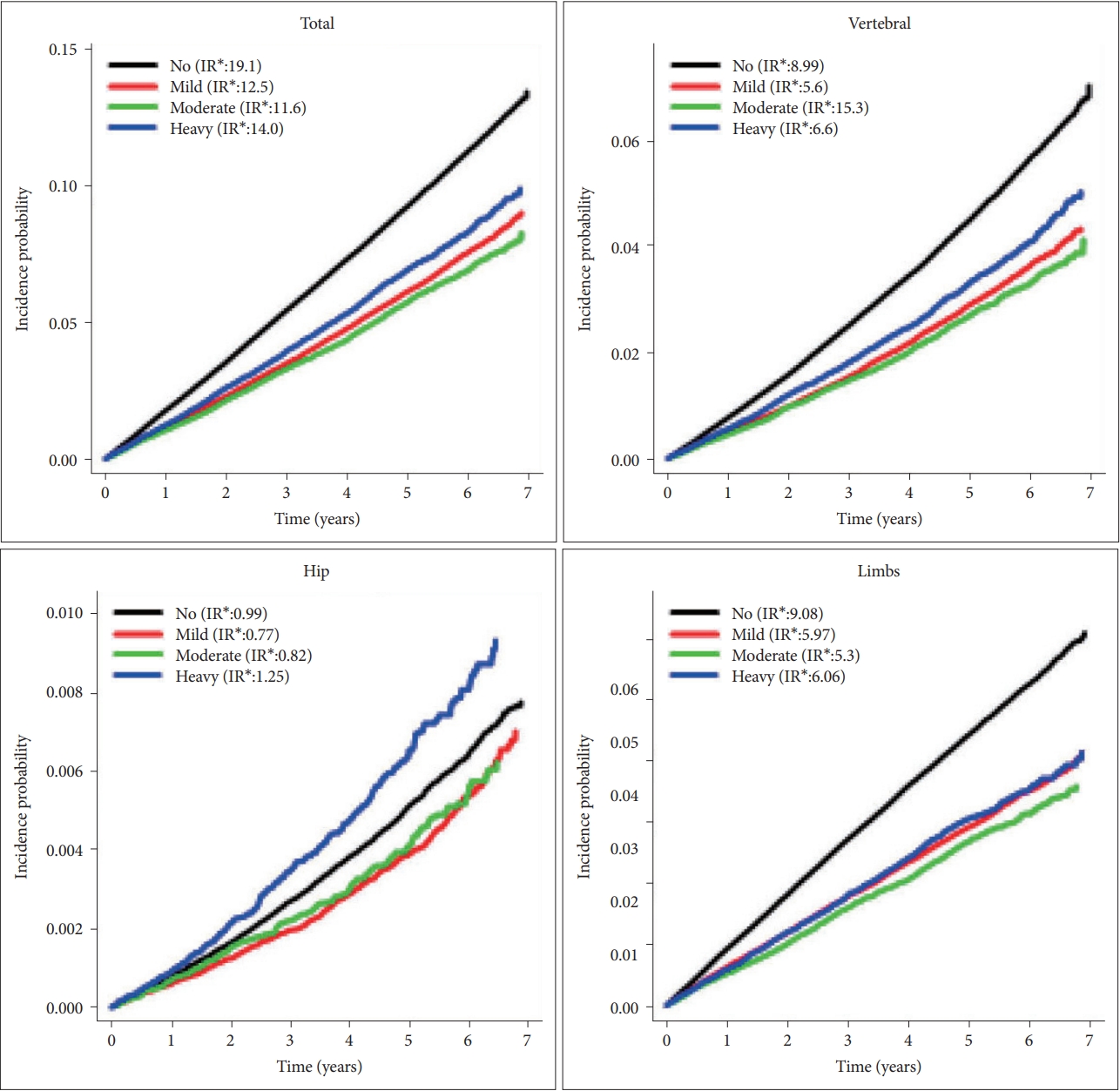
In cox regression, compared with non-drinkers, total fracture was significantly lower in the mild drinking group [adjusted hazard ratio (aHR)=0.952; 95% confidence interval (95% CI)=0.931–0.973] and higher in the heavy drinking group (aHR=1.246; 95% CI=1.201–1.294). In terms of specific fractures, similar trends were observed for vertebra. However, the risk of hip fracture decreased in both mild (aHR=0.787; 0.72–0.86) and moderate (aHR=0.773; 0.675–0.884) drinking groups, and the significance was not found in heavy (aHR=1.112; 0.979–1.262) drinking group. Lastly, risk of limb fracture showed a linear relationship with alcohol intake (Figure 2).
Fracture risk according to frequency of alcohol intake
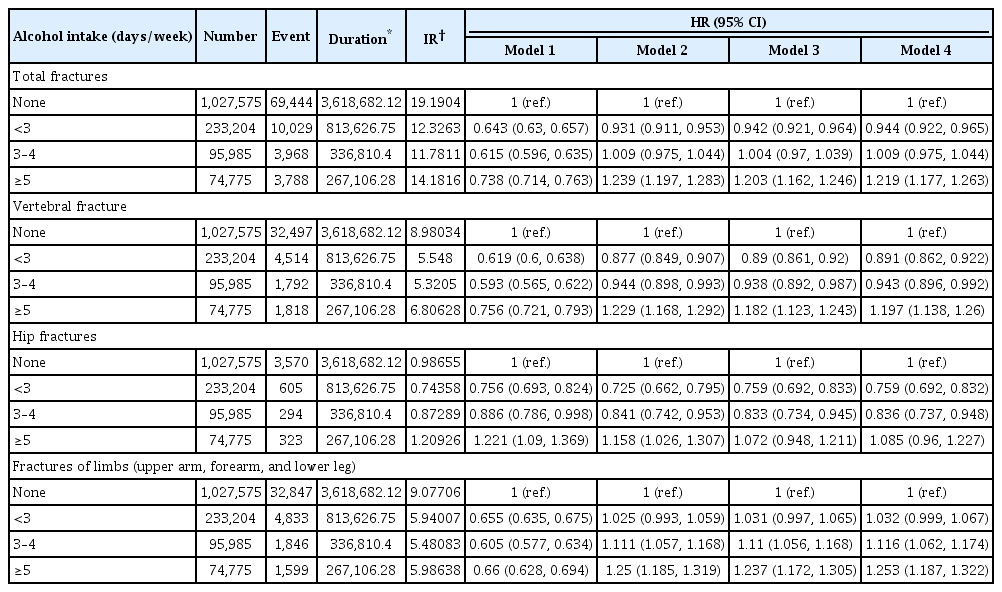
In terms of total fracture, we found a 21.9% (aHR=1.219; 1.177–1.263) increased risk in those drinking more than or equal to 5 times/week compared to none drinking group. In addition, the fracture risk significantly declined in those drinking less than 3 times/week (aHR=0.944; 0.922–0.965). This J-shaped association between frequency of alcohol intake and fracture risk were also noted for vertebra. The risk decrement from mild~moderate drinking was more pronounced for hip fracture (24% for drinking less than 3 times/week and 6% for drinking 3–4 times/week). In contrast, the risk of fracture heightened linearly as frequency of drinking increased (aHR=1.116; 1.062–1.174 for those drinking 3-4 times/week and (aHR=1.253; 1.187–1.322 for drinking 3–4 times/week) (Table 2).
Fracture risk according to amount of alcohol intake
Once again, a J-shaped association was observed for daily alcohol intake and risk of total fracture. The risk became higher from those drinking ≥30 g/day (aHR=1.15; 1.076–1.229), and we found a 47.5% increased risk in those drinking ≥60 g/day. Similar trend was observed for vertebral fractures. The risk decrement was more pronounced for hip fractures, and the significant increase was only observed for those drinking ≥60 g/day (aHR=1.446; 1.162–1.801). The risk of limb fracture increased linearly with amount of alcohol intake, and there was 54.1% increment for those drinking ≥60 g/day (Figure 3).
DISCUSSION
To the best of our knowledge, this is the largest nationwide cohort study investigating association between alcohol consumption and risk of fracture in elderly population. As expected, male ratio was lowest in none drinking group and highest in heavy drinking group. Chronic medical conditions (i.e., diabetes, hypertension, smoking, and others) related with metabolic syndrome were also higher in moderate to heavy drinking groups than none to low drinking groups, which corresponds with previous researches [24-27]. However, rate of hyperlipidemia decreased sequentially from none to heave drinking group. Increased hepatic secretion of very-low-density lipoprotein and impairment in the removal of triacylglycerol-rich lipoproteins from plasma are two important mechanisms of alcoholic hyperlipidemia, where former plays more major role [28]. With more chronic and intense alcohol intake, the hyperlipemia tends to disappear because of enhanced lipolytic activity and aggravation of liver injury [29]. Thus, inverse relationship between hyperlipidemia and alcohol intake might indirectly suggest that participants were properly allocated based on their drinking habits and intensity.
In terms of association between alcohol and total fracture, we first replicated previous findings and confirmed that the J-shaped association between alcohol consumption and fracture risk remained even in the elderly population (≥66). We found that heavy drinkers having the highest (24.6% greater) and mild drinkers having the lowest (4.5% less) risk of fracture compared with none drinkers. Similar association was observed for the weekly alcohol consumption frequency.
In addition, the fracture risk was decreased in the lowest daily alcohol consumption (<10 g/day=5.4%) group, and the risk became sequentially greater from those drinking ≥30 g/day (15%) to ≥60 g/day (47.5%).
The pathophysiological basis of alcohol consumption and fracture risk having a non-linear relationship is still obscure. Studies repeatedly showed that light to moderate drinking resulted in higher bone mineral density (BMD) and reduced agerelated bone loss [30,31]. In contrast, heavy alcohol intake was associated negative impact in bone quality, decreased BMD, and higher age-related bone loss [32,33]. Likewise, light to moderate drinkers were shown to have better health-promoting behaviors such as increased physical activity, social interactions, and a nutritious diet, which will also culminate to reduced age-related bone loss [34,35]. In contrast, heavy drinkers tended to make unhealthy lifestyle choices and have higher risk of alcohol-associated disease and altered endocrine signaling, which results in negative impact in bone remodeling [4,36-38]. Studies also showed that light alcohol drinkers had a lower risk of incident falls than non-drinkers [11]. In line with this hypothesis, a reverse U shape between physical activity and drinking groups was also observed in our cohort. The fact that history of fall was more common in none-drinking group could also be an important contributing factor. In the other perspective, large proportion of subjects in non-drinkers might already have been physically too frail to enjoy alcohol consumption. Likewise, non-drinkers were also shorter, had lower weight, and conducted less physical exercise. Thus, the higher risk of fall and fracture in the non-drinker group could have been a result of physically frailty rather than from alcohol abstinence. Further large cohort studies with longitudinal design are needed to clarify this important controversy.
Interestingly, risk pattern of alcohol consumption and fracture differed according to affected bones. For vertebral bones, a similar J-shaped association was noted between drinking group, drinking frequency, and daily alcohol consumption with that of fracture risk. However, this this J-shape was not evident, and the risk tended to increase linearly in limb fracture. Alcohol intake, even in low amount, is known to be associated with impaired judgement and poor motor control resulting in traumatic injury [39-41]. The poor body coordination might have increased with alcohol consumption intensity which resulted in linear association between alcohol intake and fracture of the limbs. Osteoporosis might have been another important cause because BMI tended to decrease linearly associated with increasing drinking intensity.
In contrast, the fracture risk decrement from mild to moderate drinking was more pronounced for hip fracture, and the risk increment did not show statistical significance even in the severe drinking group. Likewise, there was more than 20% decrement for those drinking <10 g/day and 10–20 g/day, and the significant increase of hip fracture was only observed for very severe drinkers [those drinking ≥60 g/day (aHR=1.446)]. All the participants in our study were aged 66, which can be classified as “young-old.” [42] Unlike fracture of limbs, light trauma may not have resulted in hip fracture in these “young-old” because their osteoporosis might not have been critically severe. In addition, for light to moderate alcohol drinkers, the benefits of alcohol in bone mineral density could have out weighted the risk of fracture occurring from minor traumatic events. In with our hypothesis, risk factors for hip fracture are known to change with age [8]. Studies suggested that falls and fall- related factors were most predictive of hip fracture in those who are older than ≥75, but in those who are younger [43]. Likewise, many other studies also showed that hip fractures were increased only in heavy drinking groups [10,13].
Our study has other strengths. First, we included all people aged 66, a total of 1,431,539 subjects, who received health screening from 2009–2014. Thus, besides having the largest cohort, we were able to prevent selection bias and minimize recruitment setting effect, thereby our results have higher generalizability. By including patients with 66 only, we were also able to completely remove “age” as a potential cofactor and focus on impact of alcohol consumption in the fracture incidence. In addition, we are the first to show that the fracture risk from alcohol intake could be different depending on the affected bones.
This paper also has several limitations. The intake of alcohol was based on a self-reported questionnaire. Thus, reporting bias is an important issue. Osteopenia, osteoporosis, and bone mineral density are all important risk factors of fracture, but we did not include them in the analysis. We were not able to investigate relationship among cause of fracture, fracture sites, and alcohol consumption intensity despite their potential associations. Lastly, association among gender, alcohol preference or type of alcohol, and fracture risk was not addressed.
In conclusion, we showed that there is a J-shaped association between alcohol consumption and risk of total fracture. We further demonstrated that pattern of fracture risk differed depending on the skeletal sites. Vertebra showed a similar J-shaped association between alcohol consumption and fracture risk, but the fracture risk tended to increase linearly for limbs. For hip bones, fracture risk decrement from mild to moderate drinking was more pronounced and risk increment was only noted in very severe drinkers.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1A2C2009100).
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Sheng-Min Wang, Hyun Kook Lim. Data curation: Hyun Kook Lim. Formal analysis: Hyun Kook Lim, Kyung-Do Han. Funding acquisition: Hyun Kook Lim. Investigation: Yoo Hyun Um, Dong Woo Kang, Nak-Young Kim, Hae-Ran Na. Methodology: Hyun Kook Lim. Project administration: Hyun Kook Lim. Resources: Hyun Kook Lim. Supervision: Chang Uk Lee. Writing—original draft: Sheng-Min Wang. Writing—review & editing: Kung-Do Han, Yoo Hyun Um, Dong Woo Kang, NakYoung Kim, Hae-Ran Na.