Transdermal Nicotine Patch Effects on EEG Power Spectra and Heart Rate Variability During Sleep of Healthy Male Adults
Article information
Abstract
Objective
The effect of transdermal nicotine patch on sleep physiology is not well established. The current study aimed to examine the influence of nicotine patch on homeostatic sleep propensity and autonomic nervous system.
Methods
We studied 16 non-smoking young healthy volunteers with nocturnal polysomnography in a double blind crossover design between sleep with and without nicotine patch. We compared the sleep variables, sleep EEG power spectra, and heart rate variability.
Results
The night with nicotine patch showed significant increase in sleep latency, wake after sleep onset, and stage 1 sleep; and decrease in total sleep time, sleep efficiency, and percentage of REM sleep. Also, spectral analysis of the sleep EEG in the night with nicotine patch revealed decreased slow wave activity in stage 2 and REM sleep and increased alpha activity in the first NREM-REM sleep cycle. Heart rate variability showed no differences between the 2 nights, but the low to high ratio (a parameter indicative of sympathetic nervous system activity) positively correlated with wake after sleep onset in night with nicotine patch.
Conclusion
Transdermal nicotine patch significantly disrupts sleep continuity, sleep architecture, and homeostatic sleep propensity. The overactivation of the sympathetic nervous system may be responsible for these changes.
INTRODUCTION
The role of nicotine in human sleep is not well defined. Some evidence suggests nicotine has sedating effects at low doses and alerting effects at high doses.1 A prior study found that smokers had prolonged sleep latency compared with non-smokers and that acute abstinence normalized sleep.2 In studies using transdermal nicotine patches, Davila et al.3 reported that transdermal nicotine decreased total sleep time (TST), sleep efficiency, and REM sleep time and prolonged initial sleep latency (SL) in non-smoking and obese sleep disordered breathing patients. Gilin et al.4 suggested that transdermal nicotine was associated with early morning awakening and reduced total REM sleep time in a dose-dependent fashion. Salin-Pasucual et al.5 also reported that transdermal nicotine caused sleep fragmentation and reduction of REM sleep time in non-smoking volunteers.
So far, most of the studies on the role of nicotine were based on classical sleep variables calculated from visual sleep stage scoring.234567 While visual sleep scoring is useful for characterization of sleep architecture and continuity, it is insufficient for more refined, quantitative analysis of sleep electroencephalogram (EEG).8 All-night spectral analysis of the sleep EEG provides quantitative information on the continuously fluctuating patterns of the EEG during sleep.9 A study comparing the EEG spectral power as well as the polysomnographic findings of smokers and non-smokers, found lower percentage of delta activity and higher percentage of alpha activity in the early part of sleep.10
Delta activity which is also referred to “slow wave activity (SWA)” encompasses components of the EEG signal in the frequency range of approximately 0.5 to 4.5 Hz as obtained by spectral analysis. SWA represents a physiological marker of the homeostatic facet of sleep regulation, as it increases with wake duration, peaks in early sleep, and declines in late sleep.111213 The decline in SWA during sleep can be approximated by an exponential decay and its rise in the course of waking by a saturating exponential function.11 This is evidenced by the physiological enhancement of “sleep pressure” from sleep deprivation which enhances power density in the entire delta and theta frequency range (0.5–10 Hz), whereas power in the frequency band of sleep spindles (12–15 Hz) is reduced.14151617 About the mechanisms responsible for the increase of SWA after prolonged sleep deprivation, Tononi and Cirelli18 suggested that the increase in SWA occurs because during wakefulness, many cortical circuits undergo synaptic potentiation, as evidenced by the widespread induction of long-term potentiation-related genes in the brain of awake animals.
Specific frequency bands in EKG spectral analysis are suggested to be related to parasympathetic or sympathetic nervous system activity.19 Relative power in high frequency (HF) areas, usually from 0.15 to 0.5 Hz, may refer to parasympathetic nervous system activity. Lower frequencies (LF) from 0.05 to 0.15 Hz are characteristic of both parasympathetic and sympathetic influences. Because the LF power is a combination of parasympathetic and sympathetic effects, investigators frequently infer sympathetic nervous system activity from the ratio of low (parasympathetic and sympathetic) to high (predominantly parasympathetic) (LHR) power. Nicotine is reported to increase arousal and attention and elevate heart rate and blood pressure202122 by changes in the sympathetic activity. Past studies have tried to explore the underlying autonomic mechanism of transdermal nicotine effects on human sleep by EKG spectral analysis.23242526
In the current study, in order to examine nicotine effects on homeostatic sleep propensity and autonomic nervous system function, we analyzed both sleep EEG and EKG power spectra in the nights transdermal nicotine patch compared to the nights with placebo patch. We predicted that transdermal nicotine would disrupt human sleep architecture, suppress homeostatic sleep propensity, and facilitate sympathetic nervous system activity.
METHODS
Subjects and study protocol
Sixteen nonsmoking young healthy male adult volunteers (24±1.15 years, range: 22–26) were studied with polysomnography. Inclusion criteria were as the following: 1) non-smoker males, 2) no history of mental and physical disorders at present and in the past that might influence sleep architecture. Depression was ruled out by using Hamilton Rating Scale for Depression27 and Beck Depression Inventory28 (cut-off score of 10), 3) no heavy snoring or obesity (body mass index >28), 4) no sleep disorders such as sleep apnea syndrome, idiopathic hypersomnia, narcolepsy, and periodic limb movement disorder, confirmed with overnight polysomnography, 5) no history of drug abuse, and 6) no use of medications that may affect sleep. The study was explained to them. Then, a signed consent approved by the Institutional Review Board in Yongin Mental Hospital was obtained. The subjects were asked to abstain from ethanol and caffeine for at least 3 days prior to each sleep recording and during the experiment, Breath-ethanol concentrations were measured with a Lion Alcometer S-D2 (Lion Laboratories plc., Barry, Wales, UK).
Polysomnography was conducted for three nights. The first night was for adaptation and to rule out sleep disorders. On the second night, one transdermal nicotine or placebo patch was administered in a double blind randomized crossover design. One nicotine patch contained 14 mg of nicotine. Both nicotine and placebo patches were generously provided by Sam Yang Pharmaceutical Company as the identical shape and color. Every patch was applied at 18:00 on the upper outer arm about 4–5 hours before retiring to bed. Recording started between 22:00 and 23:00. The patch was removed the next morning. After a washout period of minimum 84 hours, the third night polysomnography was carried out and the placebo or nicotine patch was administered in a counter-balanced order. Subjects were allowed to maintain their usual lifestyle except during experimental night times.
Polysomnographic recordings and digitization
Polysomnographic recordings were done with Grass model 78 polysomnograph, which included electroencephalography (EEG), left and right electro-oculography (EOG), submentalis electromyography (EMG), left and right anterior tibialis EMG, electrocardiography (ECG), snoring microphone, oro-nasal airflow, chest and abdominal respiratory movements, and arterial oxygen saturation.
Two channels of EEG were recorded at C3/A2 and O2/A1 according to the international 10–20 system.29 EOG electrode placement was done following the standard method. ECG electrode was attached at modified lead II position. The snoring microphone was attached at the surface area nearest to the vocal cord. Oro-nasal airflow was monitored with the thermocouple positioned at the mouth and the nostrils. Chest and abdominal breathing movements were monitored with impedance pneumograph applied to the chest and abdomen at the maximum excursion of breathing. Oxymeter probe was attached at the tip of the non-dominant second finger. All recordings were done at the paper speed of 10 mm/second. Behavioral monitoring was also done with infrared closed circuit camera system.
Polysomnography data were digitized and analyzed by the digital polysomnography system, developed jointly by Division of Sleep Studies in Seoul National University Hospital and Institute of Biomedical Engineering in Seoul National University using visual C++ programming language (Microsoft visual C++, version 6.0, USA) and a high performance analog-to digital (AD) and digital signal processing (DSP) card based on a transputer in order to record and process simultaneously in real time. EEG and EKG data were sampled with a frequency of 250 Hz.
Visual sleep scoring and sleep variable definition
Sleep stages were scored visually in 30-second epochs from the recording, according to Rechtschaffen and Kales criteria.30 Movement time epochs were not scored separately but were included in the wake stage.
All-night sleep variables were derived from the visual scoring of recordings using standard criteria and were grouped into 2 catogories: sleep continuity measures and sleep architecture measures. Sleep continuity measures included time in bed (TIB), TST, sleep efficiency in percent (TST/TIB×100), sleep latency (time from lights-out to the first occurrence of a stage 1 or 2 epoch), and REM sleep latency (time from sleep-onset to the first epoch of stage REM). Sleep architecture measures included duration and percentage of time spent asleep in the different stages of sleep (stage 1, 2, 3, 4, REM, and slow wave sleep, i.e. the sum of sleep stages 3 and 4).
Power spectral analysis of sleep EEG and EKG data
For each subject, one EEG derivation (C3-A2) was selected to determine the power spectra. The spectral analysis was performed with the fast Fourier transform (FFT) algorithm. The epoch length was 2 seconds (256 points), and truncating error was reduced with applying Hanning window. In addition, the values for 15 adjacent 2-second epochs were averaged to yield power density values for 30-second periods. Thus, sleep visual scores of each 30-second epoch were synchronized with power density values. The values of adjacent 0.5-Hz frequency bins were collapsed into slow wave activity (0.5–4.5 Hz; SWA), spindle frequency activity (12–15 Hz; SFA), theta (4–7.5 Hz), alpha (8–12.5 Hz), beta1 (13–21.5 Hz), and beta2 (22–30 Hz) frequency bands. Power spectral parameters were calculated within a specific period of time. Consecutive NREM-REM sleep cycles were defined according to modified criteria of Feinberg and Floyd31 by the succession of an NREM sleep episode of at least 15 minutes' duration and an REM sleep episode of at least 5 minutes' duration.
For each subject, 300 continuous beats of EKGs without artifact were chosen from both stage 2 and REM sleeps and were also used for power spectral analysis. We compared the heart rate variability including LF and HF power, LHR, very low frequency power (VLF), R-R means, and R-R variance between the nights with and without transdermal nicotine patch.
Statistical analyses
Statistical software package SPSS 11.0 for windows was used. Tests for normality of continuous measures were made with the Kolmogorov-Smirnov test in conjunction with plots of the distribution and descriptive statistics. If normality was confirmed for the measures, parametric method (paired student t-test, Pearson's correlation test) was used. If normality was not confirmed, non-parametric method (Wilcoxon signed rank test) was used.
RESULTS
The data of 1 subject who showed sleep-onset REM period (SOREMP) on the second and the third day was excluded according to the exclusion criteria.
Sleep variables derived from visual scoring
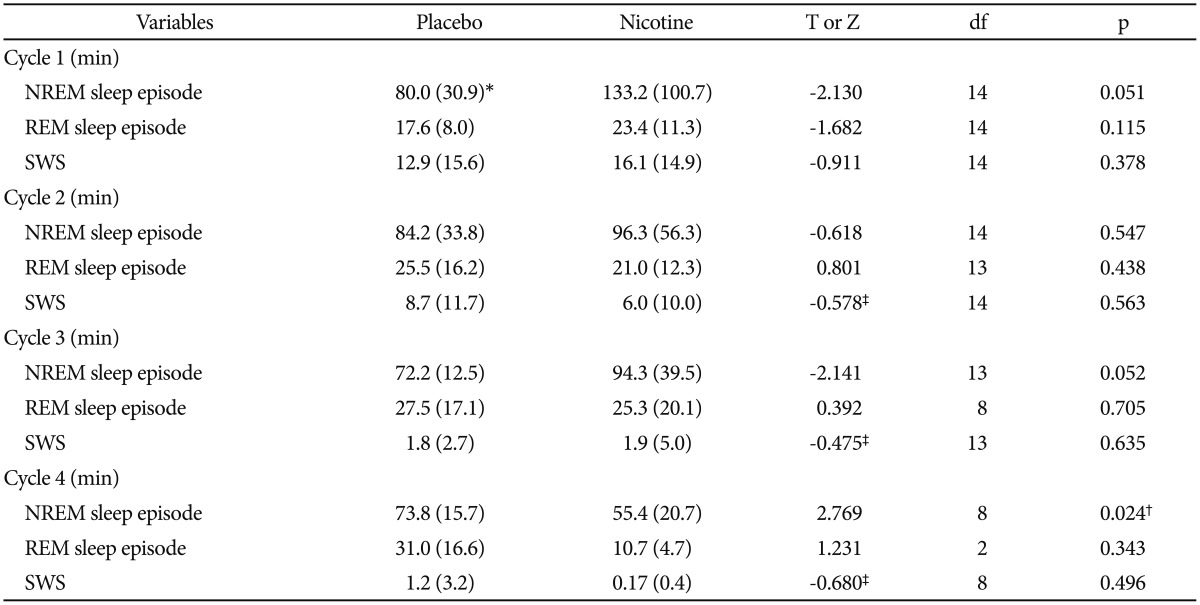
Table 1 lists the sleep parameters derived from visual scoring compared between the nights with and without transdermal nicotine patch. Among sleep continuity measures, all parameters except TIB showed significant differences between the nights. On the nights with nicotine patch, as compared to the nights with placebo patch, TST (321.8 minutes vs. 422.7 minutes, p<0.01) and sleep efficiency (72.5% vs. 91.6%, p<0.01) were decreased and wakefulness after sleep onset (WASO) (97.8 minutes vs. 23.8 minutes, p<0.01), sleep latency to stage 2 (23.7 minutes vs 9.2 minutes, p<0.01), and sleep latency to REM stage (105.1 minutes vs. 74.9 minutes, p=0.05) were increased. Among sleep architecture measures, on the nights with nicotine patch, as compared to the nights with placebo patch, percentage of stage 1 was increased (15.5% vs. 10.9%, p<0.01) and percentage of stage REM was decreased (19.7% vs. 23.4%, p<0.05). In the sleep variables for the first four consecutive cycles, non-REM sleep episode on the fourth cycle was decreased on the nights with nicotine patch compared with the placebo patch (55.4 min vs. 73.8 min, p<0.05) (Table 2).
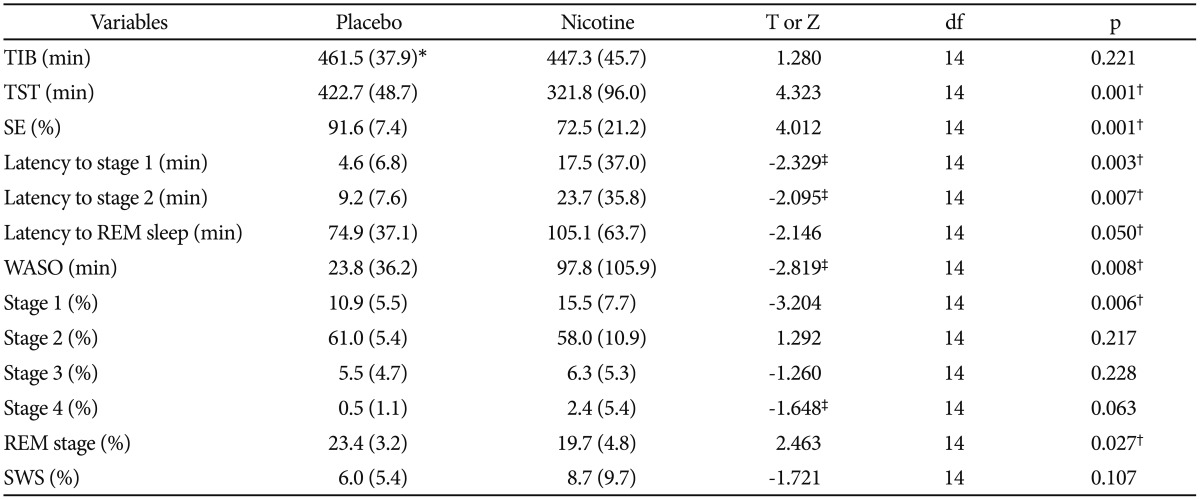
Sleep variables derived from visually-scored nocturnal polysomnography after treatment with placebo or nicotine patch
Sleep EEG spectral power
EEG power density of slow wave activity in stage 2 and REM sleep of the first 3 NREM-REM cycles were plotted relative to the corresponding total values of each stage. During stage 2 sleep, SWA was significantly lower in nights with transdermal nicotine patch compared to nights with placebo patch (t=2.442, df=14, p<0.05) (Figure 1). The difference was confined to the first cycle of the nights. For spindle frequency activity, theta, alpha, beta1, and beta2 activity, no statistical differences were observed.
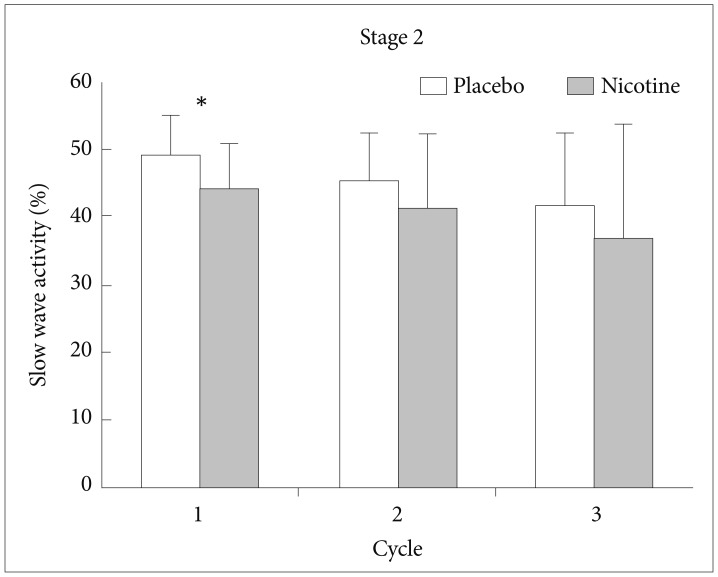
EEG power density of slow wave activity in stage 2 sleep of the first 3 NREM-REM sleep cycles. *p<0.05 by paired t-test. EEG: electroencephalogram, NREM: non-rapid eye movement, REM: rapid eye movement.
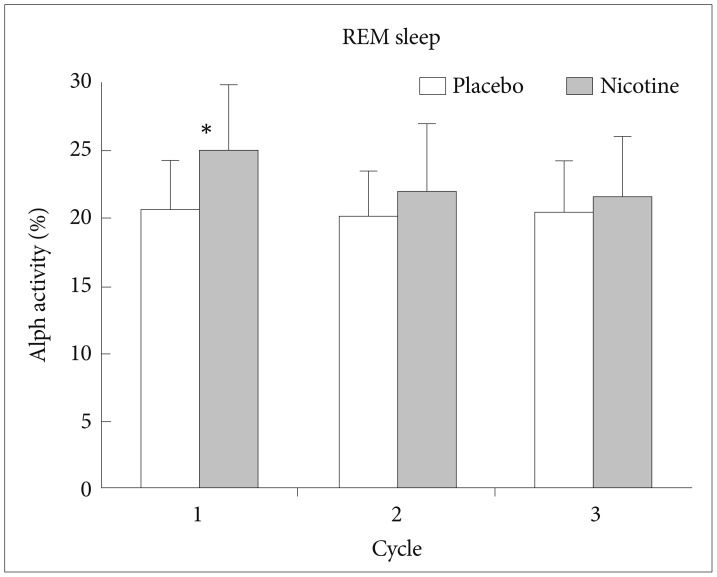
During REM sleep, SWA was significantly lower in nights with transdermal nicotine patch compared to nights with placebo patch on the first NREM-REM cycle of the nights (t=2.498, df=14, p<0.05) (Figure 2). During REM sleep, alpha activity was significantly higher in nights with transdermal nicotine patch compared to nights with placebo patch also on the first NREM-REM cycle of the nights (t=−2.673, df=14, p<0.05) (Figure 3). For spindle frequency activity, theta, beta1, and beta2 activity, no statistical differences were observed.
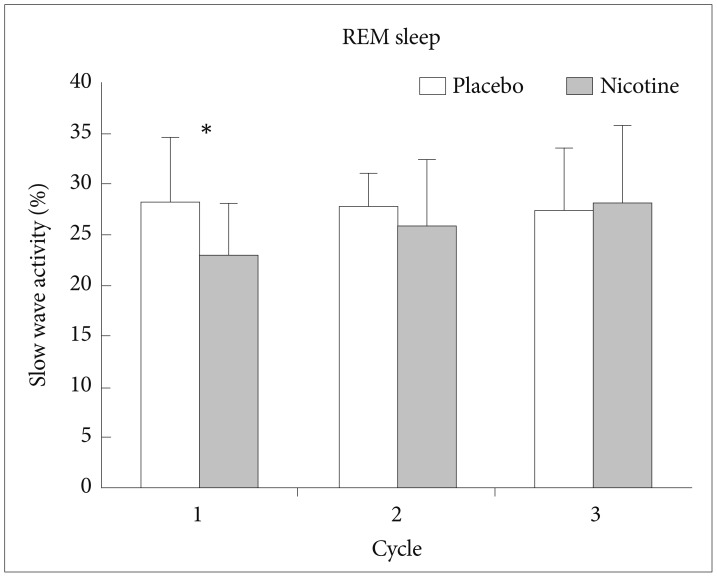
EEG power density of slow wave activity in REM sleep of the first 3 NREM-REM sleep cycles. *p<0.05 by paired t-test. EEG: electroencephalogram, NREM: non-rapid eye movement, REM: rapid eye movement.
Heart rate variability
LF and HF power, LHR, VLF, R-R means, and R-R variance in stage 2 and stage REM sleep were compared between the nights with transdermal nicotine patch and placebo patch. However, no statistical differences were observed.
LHR, indicating sympathetic nervous system activity, was 12.7±31.2 during REM sleep in the nights with transdermal nicotine patch but not significantly higher than the nights with placebo patch (8.3±15.8). We examined the correlation between LHR and other variables including TST, sleep efficiency, WASO, and SWA. Significant correlation was found between LHR and WASO during stage 2 sleep in the nights with transdermal nicotine patch (r=0.535, p<0.05) (Figure 4), but not in the nights with placebo patch. A tendency for reverse correlation between LHR and SWA during stage 2 sleep in the nights with transdermal nicotine patch (r=−0.442, p=0.099) was observed.
DISCUSSION
As we predicted, the current study shows that transdermal nicotine patch causes significant effects on sleep continuity, sleep architecture, sleep EEG and sleep EKG spectral parameters. Based on parameters derived from visual scoring, the effects of nicotine on sleep were mostly characterized by increased wakefulness and decreased TST and sleep efficiency. Moreover, a clear trend toward REM sleep reduction in the nights with transdermal nicotine patch was observed. These findings are consistent with prior studies.34
Nicotine is known to act as a presynaptic agonist to release acetylcholine from the nerve terminal.32 Acetylcholine has a key role in inducing and maintaining REM sleep.33 Therefore, it may be expected that nicotine would enhance REM sleep. However, in most of studies with non-smoking normal volunteers, REM sleep was reported to be reduced by transdermal nicotine as in our results. Only Salin-Pascual and Drucker-Colin34 reported an increase in REM sleep in both normal volunteers and depressed patients who were chronically treated with nicotine patches. They administered nicotine patch to subjects for 4 days consecutively, and found increase in REM sleep, with the exception of on the first day. Thus, whether decreases in REM sleep are limited to the acute phase of nicotine patch needs to be further clarified.
Whereas visual scoring of sleep failed to detect significant modifications in the depth of sleep, spectral analysis revealed a number of changes. Compared to the nights with placebo patch, nights with nicotine patch had significantly less SWA in the stage 2 and REM sleep of the first NREM and REM sleep cycles, respectively. We can infer nicotine suppressed homeostatic sleep propensity and disturbed deep sleep, as SWA represents a physiological marker of the homeostatic facet of sleep regulation.111213 Because SWA generally declines across successive NREMS episodes, the nicotine effects on slow wave sleep is expected to be most prominent in the first NREM cycle.
In the first REM sleep episode, alpha activity was significantly higher in the nights with nicotine patch. Endo et al.35 reported that alpha activity is reversely correlated with REM sleep propensity and alpha activity is regarded as a physiological marker for REM sleep homeostasis.36 Therefore, increased alpha activity in REM sleep in the nights with nicotine patch means can be interpreted as suppression of REM sleep propensity by nicotine.
In the current study, we could not find significant difference in sympathetic activation, measured by EKG frequency power. However, the significant correlation between LHR and WASO and the trend for reverse correlation between LHR and SWA during stage 2 sleep in the nights with transdermal nicotine patch suggests the role of sympathetic activation in promoting arousal and suppressing homeostatic sleep propensity. This is probably due to the catecholamine stimulating effects of nicotine which produces a number of physiological effects including increased heart rate and blood pressure and dose dependent increase in the secretion of prolactine and ACTH, resulting in a subsequent increase in corticosteroid secretion.202237
Our results cannot be generalized to regular smokers. The nicotine blood levels in the nights with transdermal nicotine would have increased during sleep, whereas in regular smokers, is likely to be decreased during sleep. Also, both pharmacokinetic and pharmacodynamic effects of transdermal nicotine can differ between smokers and non-smokers. Our study was not aimed to find the change of sleep architecture in smokers, so these were not the limitations of this study. Rather, our results suggest that beginning smokers can suffer from sleep difficulty. One of the limitations of this study is small sample size.
The use of transdermal nicotine patch in smoking cessation may be an effective treatment strategy. Wetter et al.38 suggested that among dependent smokers tobacco withdrawal increased objectively assessed sleep disturbance and that nicotine replacement resulted in postcessation improvements in important polysomnographic measures of sleep quality. However, a meta-analysis result showed that sleep disturbance caused by cigarette withdrawal does not seem to ameliorate with transdermal nicotine patch and paradoxically may even be increased.39 Our study results demonstrated that transdermal nicotine patch significantly disrupted sleep continuity, sleep architecture, and homeostatic sleep propensity.