Spicy Food Preference and Risk for Alcohol Dependence in Korean
Article information
Abstract
Objective
Previous studies have reported that both preference for spicy food and drinking behavior are associated with the activity of the opioid system in the central nervous system. The relationship between the preference for spicy food and the risk of alcohol dependence by comparing spicy food preference in alcohol-dependent patients vs. healthy controls was investigated. Also the association between the preference for spicy food and OPRM1 A118G was studied.
Methods
A total of 150 Korean male patients with alcohol dependence and 100 normal male control subjects were included in this study. Preference for spicy food was measured using the Food Preference Scale (FPS). DNA analysis was conducted to detect the A118G polymorphism.
Results
The mean FPS score was significantly higher in the alcohol-dependent patients (61.2±24.2) than in the normal control subjects (53.0±22.0). FPS scores differed significantly between alcohol-dependent patients and normal control subjects who had the G allele in OPRM1 A118G, but not between the two groups with the AA genotype.
Conclusion
A strong preference for spicy food can be assumed to be a risk factor for alcohol dependence, particularly in those carrying the G allele in OPRM1 A118G.
INTRODUCTION
It is well known that the mesolimbic dopamine pathway, which originates from the ventral tegmental area (VTA) and projects to the nucleus accumbens (NA),12 is closely related to drug dependence and addiction,345 and the central opioid system plays an important role in regulating the activity of this mesolimbic dopamine pathway.67 Some reports indicate that alcohol interacts with the central opioid system and leads to recruitment of the mesolimbic dopamine pathway,89 In addition, the change of the affinity of alcohol for the µ-opioid receptor caused by the A118G polymorphism may cause excessive activation of the mesolimbic dopamine pathway following alcohol consumption and play an important role in ACCESSthe risk of alcohol dependence.1011
Several studies have reported that intake of capsaicin, which is a main component of red peppers and is responsible for their spicy flavor, may increase central opioid activity.12 Lee et al.13 reported that the analgesic effect of capsaicin may be associated with increased activity of the central opioid system and that capsaicin-treated rats showed a significant increase in proopioimelanocortin mRNA expression compared to saline-treated controls at the 20-minute time point; proopioimelanocortin is a precursor of the potent opioid peptide β-endorphin. Bach et al.14 reported that β-endorphin levels in the cerebrospinal fluid (CSF) increased in the cerebellar cistern magna of white rats 45 minutes after a subcutaneous injection of capsaicin. Also, Bach and Yaksh15 reported an increase in CSF β-endorphin levels in the cerebellar cistern magna of white rats 30 minutes after an intrathecal injection of capsaicin.
Collectively, these results imply that intake of both spicy food and alcohol are associated with activation of the central opioid system. Furthermore, even though eating spicy foods is painful, some people tend to prefer spicy foods. From this point of view, we may presume that the central opioid system would be activated more in those who prefer spicy foods than in those who do not. Thus, we hypothesized that the degree of preference for spicy food can be a risk factor for the development of alcohol dependence.
There have been no previous studies on the association between spicy food preference and the risk of alcohol dependence. Therefore, the aim of the present study was to investigate the relationship between preference for spicy food and risk of alcohol dependence by comparing spicy food preference between alcohol-dependent patients and healthy control subjects. Also, we investigated the relationship between spicy food preference and a µ opioid receptor gene polymorphism (OPRM1).
METHODS
Subjects
Korean male patients who were diagnosed with alcohol dependence by psychiatric specialists in accordance with Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) criteria and who were currently inpatients in a hospital psychiatry ward or who were being treated in outpatient clinics qualified for participation in this study. Patients were excluded if they used substances other than nicotine or caffeine or had a major psychiatric disorder, such as schizophrenia, major depressive disorder, or bipolar disorder.
The final number of alcohol-dependent patients was 150. The control subjects consisted of Koreans who visited Pusan National University Hospital for a comprehensive medical examination and did not have a history of drinking more than five standard drinks per month at any time during their lives. Moreover, control subjects had to be 50 years old or older to decrease the potential that they would develop alcohol dependence in the future. The final number of subjects in the control group was 100. All subjects consented to participation in accordance with the Institutional Review Board of Pusan National University Hospital (IRB No. 2008-03).
Assessments
We examined demographic characteristics for all study subjects. We also collected information for the alcohol-dependent patients on the age at which drinking started, the age of onset of alcohol-related problems, the age of first admission to a psychiatric hospital for alcohol-related problems, the average number of drinking days and drinks per month during the 12 months prior to the present admission, the presence of a family history of alcohol dependence in a first-degree relative, and a history of severe alcohol withdrawal symptoms such as seizures, hallucinations, or delirium.
Food Preference Scale
To investigate food preferences, we developed the Food Preference Scale (FPS).16 First, fifteen medical students freely chose representative foods in each taste category (spicy, bitter, sweet, sour, and salty). Then, five foods representing each taste were chosen according to their frequency. After the respondents again identified them as being representative of each taste, we decided on three foods for each taste category in accordance with their rankings. These fifteen different foods were then randomly presented to the study subjects.
The preference for each food was measured using the visual analogue scale (VAS). Respondents identified their degree of preference on a 100 mm horizontal line that contained the phrases “don't like it” at the left end and “like it very much” at the right end. We determined the preference for each food by measuring the distance from the left end of the VAS to the indicated point in units of millimeters. Spicy food preferences were assessed by the average preference for three kinds of spicy food (pan-broiled octopus with hot pepper paste, rice cakes with hot pepper paste, and sticky noodles with hot pepper paste).
DNA analysis
We obtained approximately 10 mL of ethylenediaminetetraacetic acid (EDTA)-treated venous blood from each subject for DNA extraction. Genomic DNA was extracted from blood samples using standard methods.17 The A118G polymorphism was genotyped using the polymerase chain reaction/restriction fragment length polymorphism method published by Gelernter et al.18 Genotyping was carried out in batches for each experiment. For each genotyping batch, all 250 DNA samples were analyzed in duplicate, and no discordant genotypes were generated.
Statistical analysis
The mean preferences for the three different types of spicy food (pan-broiled octopus with hot pepper paste, rice cakes with hot pepper paste, and sticky noodles with hot pepper paste) between alcohol-dependent patients and normal control subjects were compared with independent t-tests. Also, we divided OPRM1 A118G into the AG or GG genotype group and the AA genotype group, and the mean preference for spicy food was compared between alcohol-dependent patients and normal control subjects in each genotype group with the independent t-test. We used the Window SPSS 15.0 (SPSS Inc., Chicago, IL, USA) with a two-sided significance level of less than 0.05.
RESULTS
Comparison of age and past alcohol drinking history
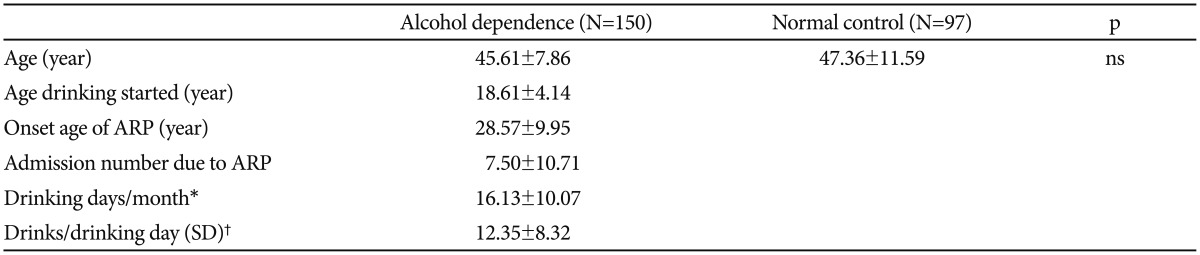
There were no significant differences between the mean age of alcohol-dependent patients (45.61±7.86) and the mean age of control subjects (47.36±11.59). In alcohol-dependent patients, the age at which drinking started was 18.61±4.14 years, and the mean age of onset of alcohol-related problems was 28.57±9.95 years. The mean number of admissions to psychiatric hospitals due to alcohol-related problems was 7.50±10.71, and the average number of drinking days per month during the past 12 months was 16.13±10.07. The average number of drinks per drinking day during the past 12 months was 12.35±8.32 (Table 1).
Differences in preference for spicy foods
The mean FPS score was significantly higher in alcohol-dependent patients (61.2±24.2) than in normal control subjects (53.0±22.0) (Table 2).
FPS score according to A118G polymorphism
FPS scores were significantly different between the alcohol-dependent patients and normal control subjects who had the G allele in OPRM1 A118G (62.2±21.0 vs. 51.3±21.5), but there were no differences between the two groups with the AA genotype (Table 3).

The association between the degree of preference for spicy foods and the risk of alcohol dependence by the µ-opioid receptor A118G gene polymorphism
However, in a subgroup comparison, there were no significant differences between the FPS scores of alcohol-dependent patients with the AA allele and those with the G allele in OPRM1 A118G. There were also no significant differences between the FPS scores of normal control subjects carrying the AA allele and those carrying the G allele in OPRM1 A118G (Table 3).
DISCUSSION
Several studies have identified a relationship among food preference, the central opioid system, and the risk for alcoholism. Garbutt et al.19 reported that a preference for sweets may be associated with the opioid system in the central nervous system. Laaksonen et al.20 reported that a preference for sweets was strongly correlated with treatment outcomes in patients given naltrexone, and that a preference for sweet foods may be used as a predictor of treatment results in alcoholics. Kampov-Polevoy et al.21 reported that a stronger preference for sweet solutions is associated with a genetic predisposition to alcoholism.
In addition to the preference for sweets, a few studies have suggested that a preference for spicy foods is associated with the opioid system and alcohol intake.2223 In spite of the fact that it is a noxious stimulant.2425 capsaicin-induced analgesia is effective for relieving postherpetic or trigeminal neuralgia, and it increases activity of the cerebral opioid system.2426 We presumed from these facts that both eating spicy foods and drinking alcohol are associated with activation of the central opioid system. In particular, the activity of the central opioid system would increase to a greater extent in those who prefer spicy food than in those who prefer less spicy food when eating spicy food or drinking alcohol. This is thought to be due to differences in the affinity of the µ-opioid receptor. The A118G polymorphism, which substitute asparagine (Asn40) for aspartic acid (Asp40), increases receptor binding affinity to endogenous opioid β-endorphin; the affinity is three times greater than that of the Asn40 allele.10 In individuals with the G allele in OPRM1 A118G, the change in the affinity of the µ-opioid receptor may cause excessive activation of the mesolimbic dopamine pathway following alcohol consumption and play an important role in the risk of alcohol dependence.1011 In conclusion a strong preference for spicy food can be assumed to be one of a risk factor for alcohol dependence, particularly in those carrying the G allele in OPRM1 A118G. And the results of this study support the notion that the degree of spicy food preference is a risk factor associated with the development of alcohol dependence.
There are several limitations to this study. First, this study does not represent the entire population of Korean alcohol-dependent individuals because the subjects were only males who were recruited from a small number of psychiatric hospitals and the ALDH assessment is not included for evaluating for control groups. Second, this study only analyzed data collected from a representative subset at one specific point in time. Third, this study is needed to evaluate the correlation of degree of spicy food preference and alcohol consumption behavior, severity, and variations depending on the person by analyzing polymorphisms of genes. Fourth, we couldn't consider the pharmacological or environmental effect such as naltrexone or hospitality to the food preference change associated with opioid system. In order to verify our hypothesis, further research is needed regarding differences in alcohol consumption, differences in type of alcohol consumed, differences in the effects of drinking, and differences in the response to opioids.
However, no previous studies have been conducted on the relationship among a preference for spicy food, OPRM1 A118G polymorphisms, and the risk of alcohol dependence. Further research will be needed to identify the relationship between other taste preferences and the effects of alcohol as risk factors for alcohol dependence. This study is significant in that it is a first attempt to identify the relationship between the risk of alcohol dependence and a preference for spicy foods, in particular, spicy Korean foods.
Acknowledgments
This study was supported by Research institute for Convergence of biomedical science and technology Grant (30-2015-013), Pusan National University Yangsan Hospital.