Factor Analysis of Delirium in Elderly, Using the Korean Version of Delirium Rating Scale-Revised-98
Article information
Abstract
Objective
This study aimed to identify the core symptoms of delirium, particularly in elderly people associated with major risk factors, using the Korean version of the Delirium Rating Scale-Revised-98.
Methods
The study sample consisted of 200 patients (mean age: 72.7±3.9 years, male: 68.5%) who had been diagnosed with delirium. Exploratory factor analysis was used to investigate the factor structure, and confirmatory factor analysis was used to evaluate the goodness of fit of the results.
Results
The results demonstrated three core domains of delirium in the elderly patients: 1) the cognitive domain (e.g., language, thought process, orientation, attention, long-term memory, and visuospatial ability); 2) the circadian domain (e.g., sleep-wake cycle and motor behavior); and 3) the short-term memory domain (short-term memory). These results were confirmed by confirmatory factor analysis.
Conclusion
The findings of this study suggest a theoretical domain structure for delirium in elderly patients.
INTRODUCTION
In order to improve understanding of delirium and its core symptoms, continuous changes have been made to the diagnostic criteria in each subsequent revision. Recently, the fifth revision of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) newly revised removed the term “consciousness” and emphasized the importance of inattention and awareness by criterion A [1]. However, attention alone as a criterion has its limits, as it demonstrated by a recent study that tried to prove its significance [2]. Other criteria need to be taken into consideration.
In this context, the DSM-5 criteria were intended to combine various associated features that support a diagnosis of delirium taking into consideration complex circumstances. Criterion B that clarified the features of rapid onset and symptom fluctuations was maintained with the exception of the additional terms, “a change from baseline attention and awareness.”[1] In particular, criterion C now describes an additional disturbance in cognition, which can include memory deficit, disorientation, language, visuospatial ability, or perception that presents with non-specific symptoms [1]. In this context, the Delirium Rating Scale-Revised-98 (DRS-R-98) is also advantageous allowing delirium to be evaluated from various perspectives [3]. Indeed, a study with the aim of demonstrating the concordance between the DSM-IV and the new DSM-5 criteria concluded that overtly strict application of the DSM-5 criteria was responsible for the accuracy in the diagnosis of delirium with detailed conditions [4].
There have been several previous attempts to clarify the core symptoms of delirium and to identify which domains are valid using Delirium Rating Scale (DRS). One of them was factor analyses that confirmed the three core domains of delirium by comparing delirious patients with normal adults [5]. And the other was a factor analysis which found that the two domains of cognition and psychosis/agitation were strongly related to delirium [6]. However, these studies were conducted in the form of a survey, targeting common adult patients and did not unilaterally consider advanced age as one of the predisposing factors of delirium [7]. Though there have been enough studies on the delirium in special circumstances like surgery or researches among older patients, these were insufficient to show the phenomenological characteristics compared with the average adults [8,9]. Recently, there was a study about the confirmatory factor analysis of the Delirium Rating Scale-Revised-98 (DRS-R-98) on a large scale [10]. However, it was a study on the verification of two-factor structure of symptoms in delirium previously elicited, using confirmatory factor analysis without the exploratory factor analysis.
Therefore, this study aimed to identify the core symptoms of delirium particularly in elderly people associated with major risk factors, hypothesizing that there are differences between delirium in elderly people and younger adults. We performed Exploratory Factor Analysis (EFA) and Confirmatory Factor Analysis (CFA) using pooled dataset [11-13], in consecutive order to establish and validate the core domains of delirium.
METHODS
Subjects
Our pooled sample comprised 536 adult patients from inpatient clinical settings [11-13]. who were diagnosed with delirium according to the fourth revision of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [14]. All of them had neither dementia nor history of dementia. Of these, the older patients aged over 80 years likely to have impaired cognitive functions that could affect the evaluation were also excluded. The reason was the fact that the prevalence of dementia which shares many characteristics with delirium is increasing with the aging. Above all, dementia appears nearly one in five in adults older than 80 years of age [15]. And In total, only 200 patients aged between 65–79 years were finally chosen for the final analysis without stringent conditions. We endeavored to obtain information concerning the particular characteristics of delirium itself without exception.
Study procedures and assessment tools
Among the various instruments used to screen and evaluate the severity and treatment outcome of delirium, only the K-DRS-98 was selected for our study. When assessing delirium using the K-DRS-98, all patients received K-DRS-98 total scores above 21.5. Our analyses used the severity items of the K-DRS-98 (13 items) but not the diagnostic items of the K-DRS-98 (total of three items).
The Delirium Rating Scale (DRS) [16] evaluated by physicians has limitations for its use in longitudinal research for several reasons. In particular, it cannot individually capture diverse phenomenological symptoms, and modified means are required to allow more accurate descriptions. The Delirium Rating Scale-Revised-98 (DRS-R-98) was created to remedy the shortcomings of the DRS [17]. The DRS-R-98 comprises two sections. 1) one section comprising three diagnostic items scored from 0 to 2 or 0 to 3, and 2) the second comprises 13 items for assessing severity scored from 0 to 3. A cut-off score for the severity items and total items was 16 and 21.5, respectively. By translating the English version into Korean using ordinary words and vocabulary, the Korean version of Delirium Rating Scale-Revised-98 (K-DRS-98) was produced and applied in this study [11].
Statistical analysis
Data were analyzed using SPSS 22.0 (IBM Corp., Armonk, NY, USA) for a general analysis and exploratory factor analysis (EFA), and AMOS 22.0 (IBM Corp.) for the confirmatory factor analysis (CFA). The author conducted a survey of 200 subjects for the EFA, and the same samples were used for CFA modeling (CFA-M) and CFA validation (CFA-V).
The EFA was performed using a principal component analysis with a Varimax rotation to determine the factor structure of the K-DRS-98. A Bartlett test of sphericity (weighted p value for χ2>0.05) and Kaiser-Meyer-Olkin (KMO) measurement (weighted value>0.6) were used to identify if the principal component analysis was appropriate for the EFA. To establish the critical value for defining which items to load onto factors, the formula 5.152/√N-2 was used, where 5.150 is twice the 1% level of significance in the normal curve, and N indicates the number of subjects (200): 5.152/14.071=0.366.
To determine if the findings gained from the EFA could form a significant model, CFA-M was conducted. The fit of the model was assessed with a χ2, coefficient χ2/df (coefficient< 2 indicates a good fit). The relative fit was evaluated using the Comparative Fit Index (CFI), the standardized rootmean-square residual (SRMR), and the root-mean-square error of approximation (RMSEA) index.
Upon consideration of the modification indices, one of the diagnostic indicators of AMOS and theoretical background, the model was modified to CFA-V. The reliability and goodness of fit were evaluated as stated above. The relationship between the core factors, three core domains, and core symptoms was assessed.
RESULTS
Sociodemographic and clinical characteristics of patients
The subjects included in this study were comprised of 200 elderly patients. Of which, 68.5% (n=137) were men and 31.5% (n=63) were women. The average age of the subjects was 72.70 years (range: 65–79 years). The principal medical diagnoses from the original datasets were categorized in accordance with the Delirium Etiology Checklist (DEC) classification system involving 13 categories [18]. The five most frequent diagnosis were: other in 68 (34%); systemic neoplasm in 50 (25%); systemic infection in 33 (16.5%); organ insufficiency 21 (10.5%); and cerebrovascular disease in 14 (7%). These are listed in Table 1.
Exploratory factor analysis
We used eight items of the DRS-R-98 to assess the severity of delirium (sleep-wake cycle, language, thought process, orientation, attention, short-term memory, long-term memory, and visuospatial ability) and united motor behavior items (motor agitation and motor retardation).
The correlation matrix evaluation indicated that the EFA of the suggested core items could be obtained (Bartlett’ test of sphericity: =453.718; p<0.000; KMO: 0.770). Eigenvalues of the three factors were >1, with the first factor=3.324, the second factor=1.228, and the third factor=1.016. The first factor was responsible for 36.9% of the variance, with the second factor 13.6%, and the third factor 11.2%. Factor loadings from the EFA of the K-DRS-98 core symptom items are listed in Table 2.
Confirmatory factor analysis modeling
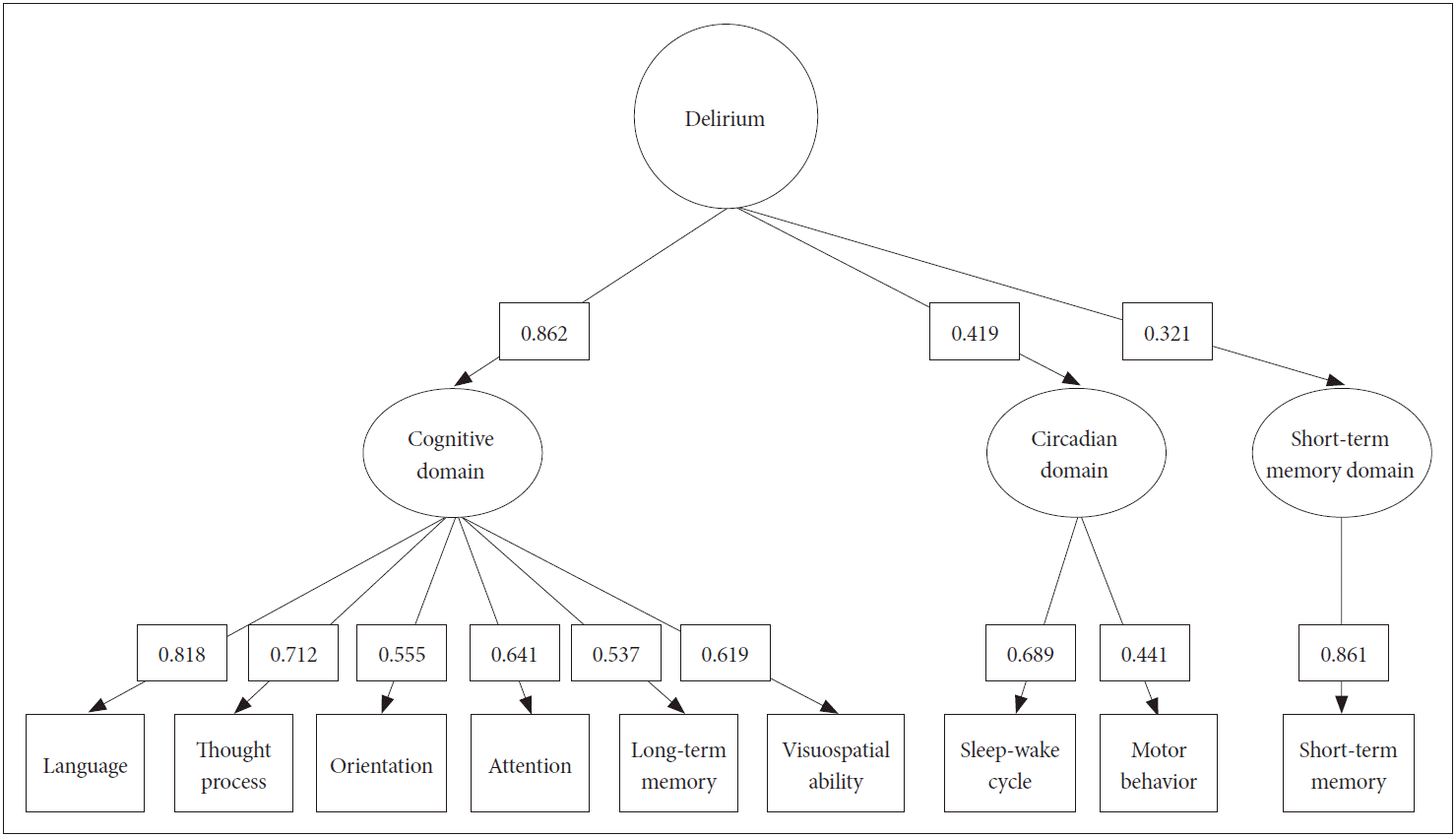
CFA for modeling the EFA suggesting the core factors of delirium is shown in Figure 1. This targeted the same samples used in the EFA. Not all loading values were elevated between the core domains and the EFA core factor, ranging from 0.86 to 0.32 (Cognitive>Circadian domain>Short-term memory). The model reliability was good (Rho coefficient=0.755), and the CFI fit index was also adequate (0.869). The other indices were as follows (χ2=81.857; df=26; p=0.000; χ2/df=3.148; SRMR=0.061; RMSEA=0.104; and 90% CI 0.079-0.130).
Confirmatory factor analysis validation
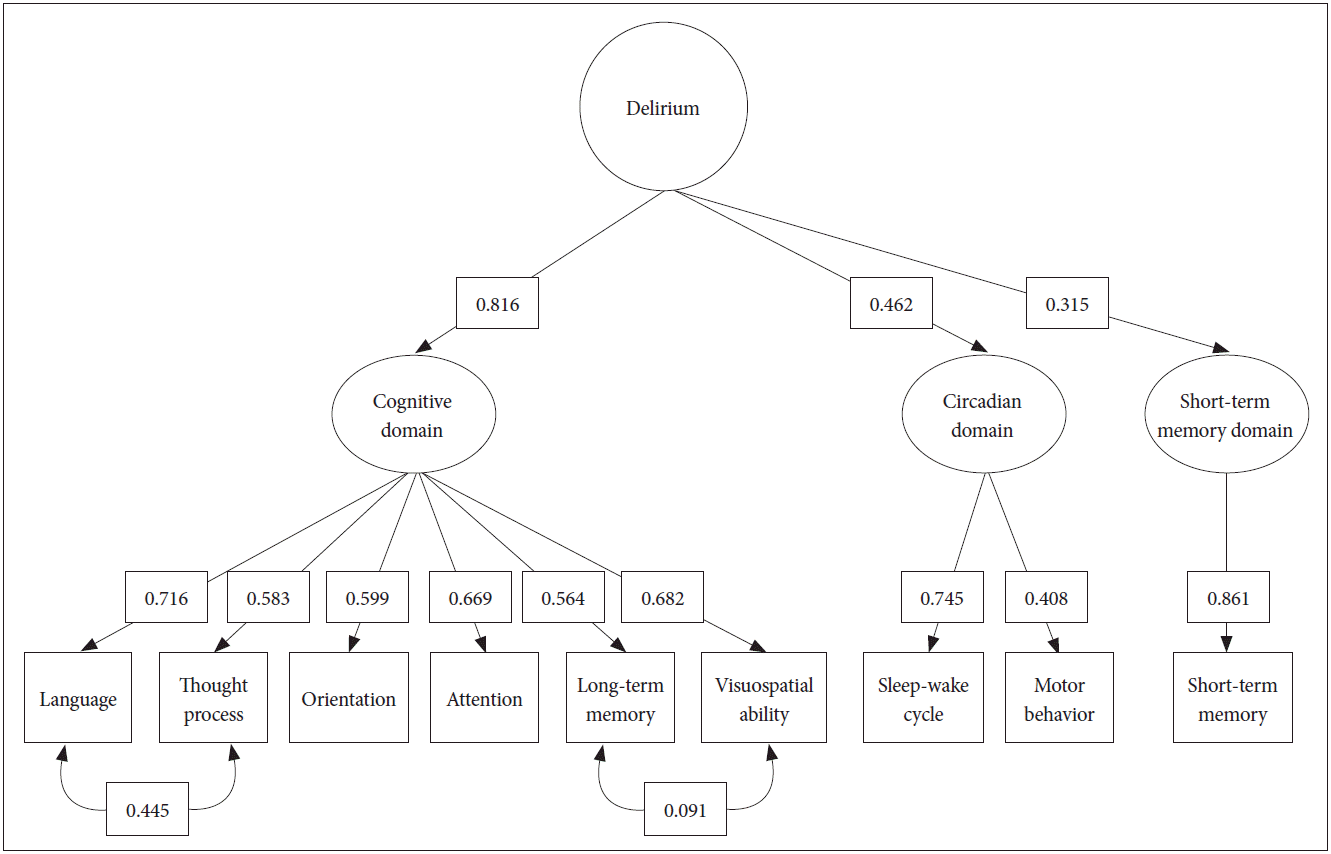
CFA for the validation of the final model of the core delirium characteristics is shown in Figure 2. Not all loading values were elevated, similar to like the preceding results of CFA-M between the core domains and the EFA core factor, ranging from 0.81 to 0.46 (Cognitive>circadian domain>short-term memory). The difference between the CFA-M and CFA-V was imperceptible. Moreover, the model reliability was good (Rho coefficient=0.824; χ2=54.134; df=24; p=0.000; χ2/df=2.256; SRMR=0.051; RMSEA=0.079; and 90% CI 0.051–0.108).
DISCUSSION
This study aimed to confirm the existence of the core domains of delirium particularly in delirious elderly patients. Three simple factors were identified by Exploratory Factor Analysis (EFA); cognitive, circadian and short-term memory domains. And these findings were also verified by Confirmatory Factor Analysis (CFA).
This trial made it possible to use a large amount of information obtained by the K-DRS-98 more effectively and reduce entries that are relatively less frequent and can distinguish the phenomenon of delirium. EFA was used to collect information simply without any loss of data [19]. To bring out the characteristics distinctly, our EFA analyses excluded perceptual disturbances, delusions, and mood lability as non-fundamental symptoms among the 13 items which represent severity in the K-DRS-98 out of 16 items (the remaining three items are used for diagnosis) [20], according to many reports so far that found certain symptoms like perceptual disturbances, delusions, and affective lability are relatively less common [21]. These uncommon and inconsistently occurring symptoms could make it harder to recognize and clarify the core features of delirium.
The EFA yielded three domains consisting of: 1) the cognitive domain; 2) the circadian domain; and 3) short-term memory domain, which accounted for approximately two-thirds of the K-DRS-98 variance. Meagher et al. [22] and Franco et al. [6] reported that sleep-wake cycle abnormalities and inattention were the most frequent symptoms in 100 delirious patients and were different to our results. Our results showed marked importance in cognitive domains than the others. Both their data and the present findings suggest that some of the characteristics are the most significant core symptoms of delirium which contrasts with the other remaining factors. This finding is accordance with those of a previous study by Franco et al. [5]. In other words, delirium can be identified primarily from the problems with cognitive functions. But, of course, attention was not the only thing that comprises the cognitive domain.
This study has a distinguishing features compared to previous studies. The factors comprising each domain differed in some parts in that short-term memory was separate from other cognitive factors, including language, thought process, orientation, attention, long-term memory, and visuospatial ability. This finding did not mean that cognitive domain and short-term memory domain are independent each other. However, it implied that short-term memory domain might have different properties. There are many evidences to show that short-term memory and long-term memory are not the distinct categories of cognitive domains distinguished from the time lag after the events only. It is supported by previous studies on short term memory and long-term memory that suggested the importance of considering the other respects of these two concepts as follows.
First, the properties of the population used in this study, in other words the elderly patients group is thought to be one of the possible reasons for this discrepancy. Next, a past report found that short-term memory and information process are features which develop independently [23]. In the past, several perspectives have been presented regarding the interrelation between short-term and long-term memory as summarized by system-based models and state-based models (for a recent overview, Underwood [24]) [25,26]. The former argument states that the storage sites between the two types of memory differ, and the latter focuses on whether they are activated or not. Another study also argued that short-term and long-term memory are essentially separate by demonstrating that short-term memory does not require the process of forming associations through a series of experiments [27]. In this respect, our results are in a position to support past studies which have tried to distinguish between these two types of memory. In addition, previous studies have shown that short-term and long-term memory are controlled by separate systems in patients who have a brain injury and exhibited the features of damaged short-term memory but intact long-term memory and vice versa [25,28]. Moreover, it is widely known that there is a link between attention and short-term memory as momentary attention plays a central role in absorbing information likely to be overlooked [29]. For several decades, there were multiple studies that discussed the relationship between attention type and aging [30]. However, it was difficult to form these arguments into the conclusion that all aspects of attention are affected by aging.
We performed a CFA to evaluate the structure of the factor results hypothesized by the EFA. By focusing on whether the extracted factors represent an original group who are delirious, both the CFA-M and CFA-V analyses were conducted. As a result, the cognitive domain was highly correlated with 0.86 in CFA-M and 0.82 in CFA-V respectively. In contrast, the circadian domain and short-term memory domain exhibited a relatively weak relationship.
The limitations of this report include that only consulted inpatients were included in the analysis. Therefore, if clinicians from each department did not assume a state of delirium, some patients may have been missed or underestimated. For this reason, datasets collected in this manner may not be applied to the general elderly population. In addition, there could be innate defects in considering dementia as exclusion criteria, for reasons of pooled data based on the clinical information from the caregivers like patient’s family and friends only. Because the results can be fairly affected, a diagnosis of dementia should be carefully considered. Another limitation is the cross-sectional nature of our data, which was not followed up after the first evaluation at the time of the onset of delirium and the assessment of transient symptoms was also not performed. This result indicates that even if changes occurred after the initial assessment, it was impossible to make full use of these sources. Finally, we did not consider the differences caused by several raters, thus introducing issues concerning inter-rater reliability. However, it was not considered to be major concern that there are several studies that performed on a larger scale using data generated from all parts of the world. Moreover, there is a general concern about the collected data because the applied criteria was not associated with an absolute standard of time due to an unavoidable lapse in memory for each evaluator.
An accurate diagnosis of delirium that has various aspects requires clarifying the core symptoms of it essentially. We should maintain the current efforts to intervene in the general population by refining the evaluation methods and conduct follow-up studies in the future. However, the findings in this report provide a framework and a direction for further research.