Recent Updates on Electro-Convulsive Therapy in Patients with Depression
Article information
Abstract
Objective
Electro-convulsive therapy (ECT) has been established as a treatment modality for patients with treatment-resistant depression and with some specific subtypes of depression. This narrative review intends to provide psychiatrists with the latest findings on the use of ECT in depression, devided into total eight sub-topics.
Methods
We searched PubMed for English-language articles using combined keywords and tried to analyze journals published from 1995–2020.
Results
Pharmacotherapy such as antidepressants or maintenance ECT is more effective than a placebo as prevention of recurrence after ECT. The use of ECT in treatment-resistant depression, depressed patients with suicidal risks, elderly depression, bipolar depression, psychotic depression, and depression during pregnancy or postpartum have therapeutic benefits. As possible mechanisms of ECT, the role of neurotransmitters such as serotonin, dopamine, gamma-aminobutyric acid (GABA), and other findings in the field of neurophysiology, neuro-immunology, and neurogenesis are also supported.
Conclusion
ECT is evolving toward reducing cognitive side effects and maximizing therapeutic effects. If robust evidence for ECT through randomized controlled studies are more established and the mechanism of ECT gets further clarified, the scope of its use in the treatment of depression will be more expanded in the future.
INTRODUCTION
Electroconvulsive therapy (ECT) is considered as an effective therapeutic option for major depression with estimated response rates of 80–90% [1]. The mortality rate was significantly lower in the ECT group compared to the inadequate antidepressant treatment group and non-treatment group [2]. The ECT group also had significantly fewer suicide attempts than the antidepressant treatment group regardless of previous suicide attempts [3].
A meta-analysis study included eighteen trials that compared ECT with drug therapy in depression and showed that ECT was significantly more effective than was pharmacotherapy, concluding that ECT can be an effective short-term treatment for depression [4]. A report from the Consortium for Research in ECT (CORE) showed that optimized ECT results in a remission rate of 80% or more in severely depressed patients, though relapse seems to be frequent after the end of treatment [5]. ECT has been suggested as preferential treatment for depression under specific presentations, such as postpartum depression, psychotic depression with or without catatonic features, and severe suicidal ideation or attempts [6].
This review aims to provide psychiatrists with recent updates on ECT in patients with depression, focusing on eight sub-categories: maintenance therapy for preventing relapse after ECT; the efficacy of ECT in treatment-resistant depression; in depression with suicide risk; in geriatric depression; in bipolar depression; in psychotic depression; in pregnancy with depression or postpartum depression; and mechanism of ECT.
METHOD
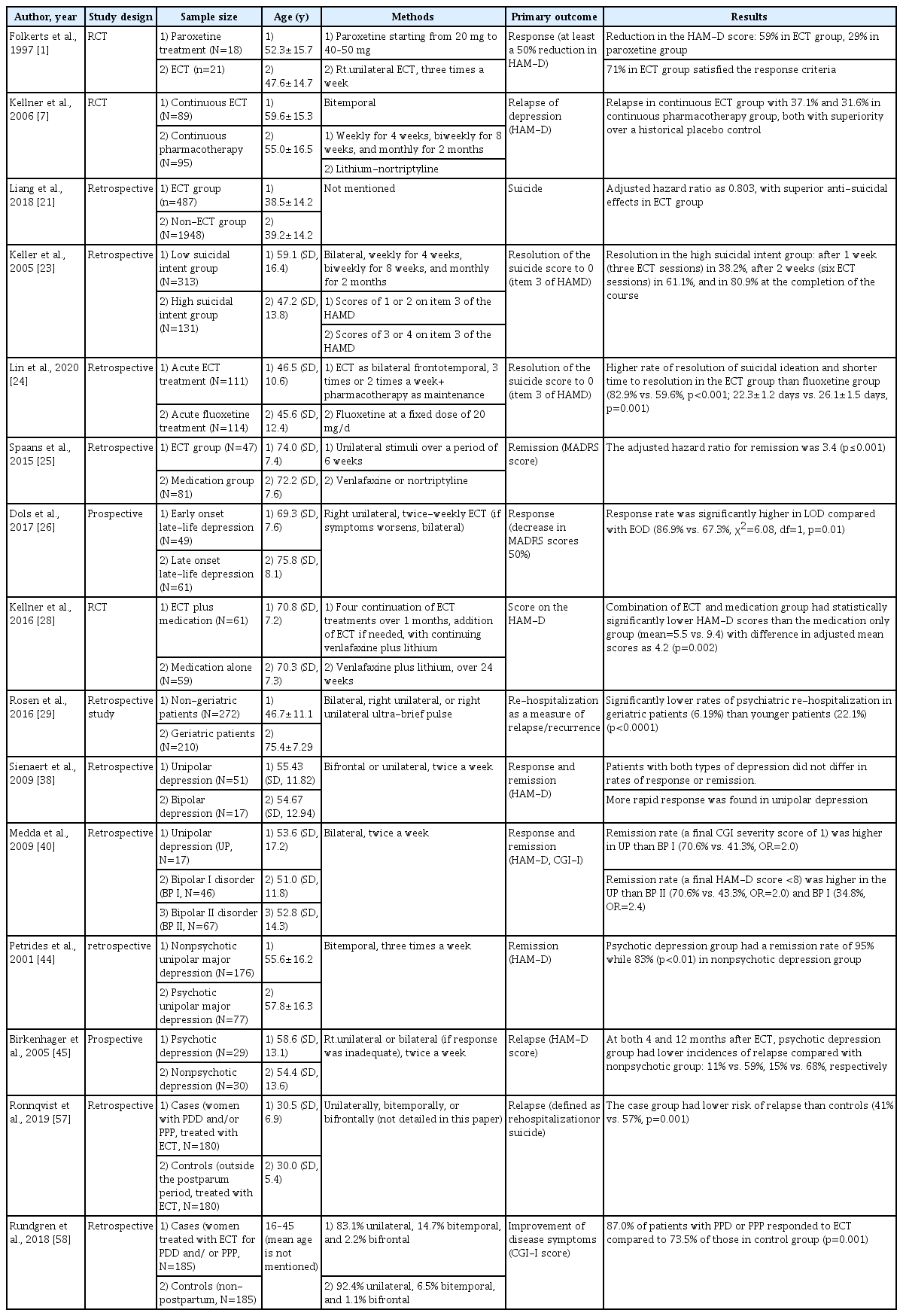
We used the following search terms on PubMed: “Electroconvulsive” OR “ECT” or “Shock therapy” and “depression” OR “depressive disorder” OR “depress” with the addition of the term reviewed later according to the subcategories (“mechanism,” “neurobiological,” “neurophysiological,” “relapse,” “continuation,” “maintenance,” “pharmaco,” “treatment resistant,” “suicidal,” “suicide,” “elderly,” “geriatric,” “late-onset,” “bipolar,” “postpartum,” “peripartum,” “perinatal,” “pregnancy”). The results of comparative studies are listed in Table 1.
RESULT
Maintenance therapy for preventing relapse after ECT
Continuation or maintenance ECT is widely accepted because of its effectiveness in preventing relapse or recurrence of depression. In a study from the CORE, continuation ECT (C-ECT) was compared with pharmacotherapy (C-Pharm) for preventing relapse after ECT in major depressive patients, of whom 37.1% experienced disease relapse in C-ECT and 31.6% in C-Pharm, with both groups showing better results than the placebo control [7]. In a meta-analysis study, four C-ECT samples (n=146) showed a 37.2% relapse rate at a six-month follow-up, with no significant difference from the relapse rate of 37.7% among modern-era pharmacologically treated patients [8]. In a recent meta-analysis study that included five studies with 436 patients, continuation and maintenance ECT with pharmacotherapy showed fewer relapses and recurrences than did pharmacotherapy alone within a year after successful completion of ECT [9]. A meta-analysis study reported that the relapse rate within one year following ECT exceeded 50%, and when placebo only was given to the patients after the end of ECT, the relapse rate reached 84% [8,10]. Although results from randomized controlled studies are needed for more rigorous evidence, maintenance ECT alone or combination with medication seems to be a reasonable treatment option for preventing relapse after ECT.
ECT on treatment-resistant depression
Treatment-resistant depression (TRD) is usually defined as depression that does not remit or respond to the use of two or more antidepressants for a sufficient period of time [11]. The response rate to antidepressant treatment among patients with TRD is less than 17%, but the response rate to ECT is reported to be between 50% and 60% [12]. When 39 subjects with TRD were randomized into the paroxetine treatment group or the ECT group, 75% of the patients in the ECT group satisfied the response criteria which is defined as least a 50% or more reduction of scores in Hamilton Depression Rating Scale (HAMD) [1]. The results from a naturalistic study that examined 38 severely depressed patients who received ECT demonstrated that the average of 5.4 medications failed before ECT, 65.8% of the subjects responded to treatment after completion of ECT, and 53.3% reached remission [13]. In clinical guidelines established by the Canadian Psychiatric Association, ECT was recommended as a first-line treatment with evidence level 1 for TRD [6] and the practice guidelines from Roya Australian and New Zealand College of Psychiatrists suggested ECT as an option for TRD early in the treatment algorithm [14]. Magnetic seizure therapy (MST) was introduced as another type of convulsion therapy. MST is similar to ECT in that it causes generalized seizures, except that it uses high-frequency repetitive transcranial magnetic stimulation instead of direct electrical current [15]. There is a growing body of evidence that recovery of orientation after MST is quick with limited cognitive side effects [16], but it needs to be compared with ECT in randomized condition to prove its efficacy in major mood disorder [17,18].
ECT on depression with suicide risk
ECT is favored when an acute antidepressant effect is required, such as when treating major depressive disorder (MDD) accompanied by severe suicidality [19]. A rapid reduction of suicidal ideations after ECT and repetitive transcranial magnetic stimulation has been reported in depressed patients [20].
Limited clinical trial data exist on ECT’s short-term or longterm effects on suicide risk. The uncontrolled studies provide evidence that ECT has an acute positive effect on suicide risk but there seems to be insufficient evidence for a long-term effect on suicide risk [19]. A nationwide retrospective cohort study included in-patients with unipolar or bipolar disorder who completed ECT and were matched with controls with psychopharmacotherapy. The patients treated with ECT had 19.7% lower risk of suicide compared to those in the control group, showing the superior anti-suicidal effect of ECT [21]. The 96 suicide victims with a primary severe depression were matched with the same number of controls from the same diagnosed sample of 1,206 patients. The suicide attempt rate was the lowest at 2% after ECT plus pharmacotherapy, 8% after ECT, and 20% after sometimes receiving antidepressant pharmacotherapy [22].
In a study of comparing continuous ECT and continuous pharmacotherapy, the expressed suicidal intent of the patients who received continuous ECT were evaluated based on the baseline suicidal intent score. In subjects with high suicidal intent [scores of 3 to 4 on item 3 of the Hamilton Depression Rating Scale (HAM-D)], 38.2% showed a decrease in score to 0 after a week (three ECT sessions), 61.1% did after 2 weeks (six ECT sessions), and 80.9% did at the completion of all ECT sessions with rapid tranquilizing effect [23]. In another comparison study on the efficacy of ECT on suicidal ideation, patients treated with ECT (n=111) had a significantly shorter time to alleviation of suicidal ideation than did patients treated with fluoxetine (n=114) for acute treatment phase. In the three month follow-up period, no significant difference was found in the time to relapse of suicidal ideation between the two groups [24]. The long-term efficacy of ECT on reducing suicidal ideation is unclear but it may be beneficial and even powerful for rapidly relieving suicidal ideation.
Efficacy of ECT on geriatric depression
Depressed elderly patients who received ECT had a mean time to remission of 3.1 weeks, which was earlier than 4.0 weeks of the medication treatment group, and had a significantly higher remission rate [25]. Post ECT, patients with late onset (≥55 years) late-life depression showed a higher response rate of 86.9% compared to those with early onset (<55 years) late-life depression who had response rate of 67.3% [26]. Recently, the Prolonging Remission in Depressed Elderly (PRIDE) study investigated the efficacy of right unilateral ultra-brief pulse (R-UBP) ECT with venlafaxine for the treatment of geriatric depression in phase I; remitted patients were randomly assigned to receive six-month pharmacotherapy or pharmacotherapy plus ECT in phase 2. In phase I, 61.7% of the patients were remitted, 74.3% of the patients reached remission within 3 weeks (nine or fewer treatments), and 70% of the patients met remission criteria [27]. In phase 2, at six months, the ECT combined with medication (venlafaxine plus lithium) group showed statistically significantly lower HAM-D scores and rated more on “not ill at all” item on CGI-S than did the medication group [28]. In terms of maintenance ECT, MDD patients (average age of 57) were randomly assigned to either a maintenance ECT group (n=89) or continuous pharmacotherapy group (n=95); Significant differences were not found in relapse rates and time to relapse between the two groups [7].
When psychiatric re-hospitalization rates of geriatric patients were compared to those of non-geriatric patients after the end of ECT, geriatric patients achieved lower rates of psychiatric re-hospitalization than did non-geriatric patients (6.2% vs. 22%) [29]. Based on the literature to date, maintenance ECT is well tolerated and seems as effective as continuation of medication after successful completion of ECT in elderly patients with severe depression [30]. Despite concerns about negative effects on cognitive functioning, such effects of ECT in geriatric depression were temporary and limited, with improved cognitive outcomes in unilateral ECT [31]. The PRIDE that investigated the neurocognitive effects of ECT in elderly patients with MDD demonstrated only phonemic fluency, complex visual scanning, and cognitive flexibility were qualitatively decreased from low average to mildly impaired [32]. Also, the course of dementia is not deteriorated due to ECT and comorbidities such as depression or agitation are indications of ECT [33].
Bipolar depression and ECT
Bipolar depression is known to have higher rates of medication non-response than that of unipolar depression, and the recurrence rate of a depressive episode is high, reaching 60% in the first 2 years and 75% in 5 years [34,35]. In patients with bipolar disorder, more than 70% of suicide attempts and suicide deaths occur during depressed periods [6]. Although ECT is usually sought as a last resort for bipolar depression, a meta-analysis study on the efficacy of ECT in bipolar depression report that response and remission rates of ECT in bipolar depression were not inferior to those in MDD [36].
A register-based observational study included data of all patients with bipolar depression treated with ECT from 2011 to 2016; 80.2% of patients reached a response and older age was associated with a higher response rate [37]. Efficacy and speed of response following ECT were compared between 51 unipolar and 13 bipolar depressive disorder patients. The results showed that the remission and response rates were not significantly different, but patients with unipolar depression presented a more rapid response [38]. However, contrasting results also exist. In a retrospective study, patients with unipolar depression tended to show greater improvement than the patients with bipolar depression [39]. When 17 patients with unipolar depression were compared with 113 patients with bipolar I or II disorder, the unipolar depression group showed a higher remission rate after ECT than either bipolar I or II disorder group [40].
However, the efficacy of ECT in bipolar depression is supported by more study results. A meta-analysis study conducted for the purpose of identifying the relative efficacy of ECT in unipolar and bipolar depression, included six cohort studies and showed that the efficacy of ECT was similar in both types of depression (OR=1.08) [41]. Another systematic review and meta-analysis study reported similar results, which included nineteen articles; a similar remission rate was found in both MDD and bipolar depression patients, although a statistically higher response rate was documented in patients with bipolar depression [36].
Psychotic depression
Psychotic depression is accompanied by psychotic symptoms such as delusions or hallucinations. Epidemiological studies of psychotic disorder presented a prevalence of psychotic depression ranging from 0.35% to 1% and association with higher rate of recurrence, decreased quality of life, higher mortality rates, and higher risk of suicide [42,43].
The expert opinions are inconsistent on the optimal treatment for psychotic depression, and the contents from nine international treatment guidelines are even contrasting. Five out of nine guidelines [the American Psychiatric Association (APA) 2010, Canadian Network for Mood and Anxiety Treatment (CANMAT) 2009, the Texas Medication Algorithm Project (TMAP) 2008, the Danish Board of Health (DNBH) 2007, the World Federation of Societies of Biological Psychiatry (WFSBP) 2020)] regard ECT as an equally appropriate first-line treatment for psychotic depression [42].
Remission rate after application of ECT in psychotic unipolar depressed patients (n=77) was compared to those in nonpsychotic depressed patients (n=176). The overall remission rates assessed by change in scores of HAM-D were 87% in the patients who completed the study, 95% in the psychotic depression group, and 83% in the non-psychotic depression group, indicating that there was a robust and quicker improvement in psychotic patients [44]. In 59 depressed patients (29 with psychotic features), the risk of relapse was prospectively assessed at one year after successful ECT. Patients with psychotic depression had significantly lower rates of relapse at one year and also at four months after the completion of ECT compared to non-psychotic patients [45]. Although the evidences are limited, ECT appears to provide favorable outcomes to patients with psychotic depression.
Depression in pregnancy and postpartum depression
Depression is the most common mental disorder during pregnancy and it is estimated that approximately 9% of pregnant women suffer from an episode of MDD [46]. As many as 50% of pregnant women get depressed after giving childbirth [47]. In a study conducted among American women in 2005, 9.1% of pregnant women and 10.2% of postpartum women met diagnostic criteria for a major depressive episode [48]. Although the evidences on effectiveness of ECT in pregnant women are mostly based on the results of case reports and there is no prospective controlled study, results from previous studies indicate that use of ECT is generally safe during the entire period of pregnancy [49,50]. Maternal changes such as decreased utero-placental perfusion, risk of aspiration, compression on aortocaval system during pregnancy and other comorbidities and the possibility of fetal heart rate change may need to be considered, but serious adverse events caused by ECT during pregnancy seem to be uncommon [51].
In a retrospective study, the efficacy and safety of ECT in 33 (n=19 MDD; the rest=bipolar disorder or schizophrenia) pregnant patients were evaluated. In MDD patients, a complete response rate assessed by change in score of Clinical Global Impression-Severity scale (CGI-S) or HAM-D was 84.21% and a partial response rate was 15.78% [52]. In one case review of ECT in pregnancy from 1941 to 2007, the efficacy data were available in 68 out of 339 cases. In the 37 cases diagnosed with either MDD or depression, 26 reached remission and five reached partial remission, with overall 83.8% achieving at least partial remission. The risks of adverse events were low [53]. Significant adverse outcomes were not reported except one case of early childbirth and pes ekinovarus deformity in a newborn which was most probably not associated with ECT [54]. Given current knowledge, although there may be some fetal complications such as self-limiting fetal cardiac arrhythmia, or early delivery and maternal complications such as vaginal bleeding, uterine contraction, abdominal pain, or changes in blood pressure, those complications are generally mild and curable [55]. Therefore, ECT is relatively safe and effective as an alternative treatment option for depression during pregnancy.
180 patients with postpartum depression and/or postpartum psychosis (0.25 to 0.6 per 1,000 deliveries, with manic, mixed or psychotic symptoms) [56] received ECT within six months after delivery and were compared to the same number of depressed patients that received ECT and were not in postpartum period. Percentages of patients who experienced relapse after 6 months, 1 year, and 2 years were lower in the postpartum group than those in the control group [57]. In another population-based study, improvement of postpartum depression and psychosis after ECT was compared to that in a matched comparison group (not within the postpartum period). 185 subjects were included in each group, and improvement at one week after ECT was evaluated using the Clinical Global Impression-Improvement scale (CGI-I). The postpartum group responded more to ECT at 87.0% than did the comparison group at 73.5% [58]. In one systematic review and case report, eight cases and eight studies were included. Although most of the patients were diagnosed with postpartum psychosis, all studies concluded that ECT is effective in the postpartum period with strength of good tolerability, fast response, and allowance for breastfeeding [59].
The neurobiological mechanism of ECT
Gamma-Aminobutyric acid (GABA) has been suggested to mediate anticonvulsant effect of ECT, supported by rise in seizure threshold during the course of ECT [60]. GABAA receptor mRNA in the hippocampus and cerebellum, GABAB binding in rat brain after repeated electroconvulsive seizures (ECS, an analogue of ECT in animal model), GABA concentration in the occipital cortex were increased [61-64]. Because elevated glutamate levels were found in the different brain areas of depressed patient [65], a glutamate-normalizing action of ECT has been suggested [66].
ECT seems to be associated with transiently increased dopamine receptor binding and neurotransmission [67], decreased α2-adrenoceptor as a result of sustained release of noradrenaline [68], increased expression of neuropeptide Y (NPY) mRNA and peptide release in cortex as well as hippocampus, and increase of thyrotropin-releasing hormone (TRH) [69]. Like antidepressants, ECT seems to reduce and further down-regulate 5- HT2 receptors in the brain70 but the findings are inconsistent [71,72].
Relationship between the activated immune system and the effects of ECT is supported by temporarily increased expression of inflammatory cytokine genes (such as IL-beta, IL-6) [73] and normalization of high cortisol levels which are associated with inhibition of neurogenesis, gliogenesis, and atrophy of structures such as hippocampus [74]. However, chronic application of ECT shows decreased immune system activity [75].
In terms of neurophysiological change, an increase in regional cerebral blood flow is observed in the brain during a seizure [76]. During ECT or immediately after ECT, regional differences in blood flow were also documented [77]. After ECT, metabolism decreased in the frontotemporal neo-cortical areas while it increased in the right medial temporal structures including amygdala and pons [78], though research findings on post-ECT reduction in absolute levels of blood flow are inconsistent [76,79].
ECT also transiently increases the permeability of the blood-brain barrier and generates changes such as increase of neurotrophic factor, angiogenesis, and neurogenesis after multiple applications of ECS [80]. Brain-derived neurotrophic factor (BDNF), known as a nerve-growth factor that mediates neuronal growth, proliferation, repair, and survival, has been widely studied for understanding the therapeutic effects of ECT and has proved to be increased after ECT [81,82]. Expression of vascular endothelial growth factor (VEGF), glial cell-line derived neurotrophic factor (GDNF) and basic fibroblast growth factor-2 (FGF-2) seems to be increased after ECT or ECS [83-85].
In a model of psychosis in mice, ECS seems to resemble atypical antipsychotics with differential effects on dopaminergic pathways [86] and neurodevelopmental hypothesis is supported with increased neuroprotective molecules such as BDNF and proliferation of hippocampal progenitor cells [87]. Compared with depression, immune system and inflammation hypothesis of psychosis is not clear with few study results.
DISCUSSION
ECT has been in use since the 1920s, but its use has been declining for some time because of negative perceptions and antipsychotic drugs were introduced one after another. During the ECT procedure, there can be cardiovascular changes such as increased blood pressure, increased heart rate or arrhythmia, anesthetic side effects such as aspiration and respiratory suppression, and musculoskeletal side effects, but safety is increasing due to improvement of ECT technique and anesthesia technique [88]. As psychiatric side effects, temporary disorientation and postictal delirium may occur, and may be accompanied by anterograde or retrograde amnesia, but usually temporary and recovers over time [89]. Recently, it is evolving toward reducing cognitive side effects and maximizing therapeutic effects. During the ECT procedure, the location of the electrode and the physical properties of the stimulation influence the seizure threshold, which is related to the therapeutic effect and cognitive adverse effects. Generally, right unilateral ECT gets less severe cognitive impairment than bilateral ECT but needs a high dose (6 times seizure threshold) for the efficacy of treatment [4]. Relative high dose compared to bilateral ECT (1.5–2.5 times threshold) can offsets the advantage of unilateral ECT [90]. Clinical data on the use of bifrontal ECT have suggested fewer cognitive adverse effects, but more evidence is needed to prove its superiority over bilateral ECT [91]. Theoretically, ultra-brief pulse width (0.3–0.5 miliseconds), lower pulse frequency and longer duration generates seizure with a small amount of charge. Ultra-brief pulse width unilateral ECT or bifrontal ECT has shown to be effective with cognitive preserving [92,93]. In addition to this, new methods such as magnetic seizure therapy or focal electrically administered seizure therapy, which can avoid cognitive impairment by inducing local convulsions, are also being tried [90]. The use of antidepressant during ECT may also affects seizure properties. Fluoxetine and mirtazapine are known to increase seizure duration and venlafaxine relatively decrease. Therapeutic effectiveness seems to be enhanced when combined with tricyclics, selective serotonin reuptake inhibitors (SSRIs) or mirtazapine. Although SSRIs may induce serotonin syndrome, most side effects were not serious and temporary [94].
As a mechanism of ECT, changes in neurotransmitters and hormones, changes in the physicochemical environment in the brain, and neuroplastic neuronal generation have been reported, but the link between the various research results is still unclear [95]. More evidence points towards using antidepressants after ECT to prevent recurrence, however maintenance ECT may have the same effect as drugs. In depressed patients with suicide risk, ECT has acute, and rapid tranquilizing effects. The use of ECT for depression in the elderly showed rapid treatment response and high remission rate, and was effective as maintenance antidepressant treatment to prevent recurrence. The effect of ECT in bipolar depression was not inferior to that in unipolar depression. Although the data are limited, patients with psychotic depression seem to show rapid response and low relapse rate when treated with ECT. The use of ECT in depression during pregnancy or after childbirth is effective in terms of treatment response, maintenance of remission, and safety. It is necessary to establish robust evidence for ECT through randomized controlled studies, and if the mechanism of ECT gets further clarified, the scope of its use in the treatment environment of depression will be expanded in the future.
Acknowledgements
This research was supported by the Original Technology Research Program for Brain Science through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. NRF-2016M3C7A1947307; PI HJJ), by the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIT (No. NRF-2017M3A9F1027323; PI HJJ), and by Healthcare AI Convergence Research & Development Program through the National IT Industry Promotion Agency of Korea (NIPA) funded by the Ministry of Science and ICT (No. S1601-20-1041).
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Hong Jin Jeon. Investigation: all authors. Writing—original draft: Mi Jin Park, Hong Jin Jeon. Writing—review & editing: all authors.