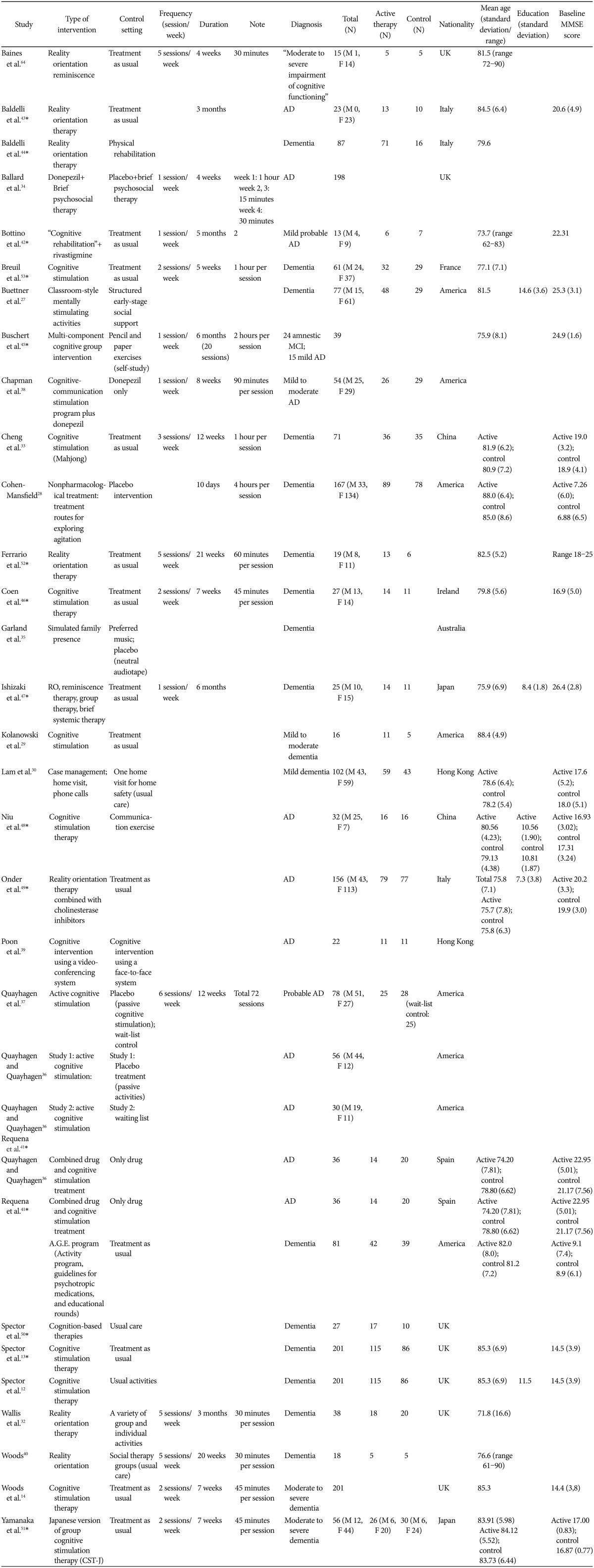
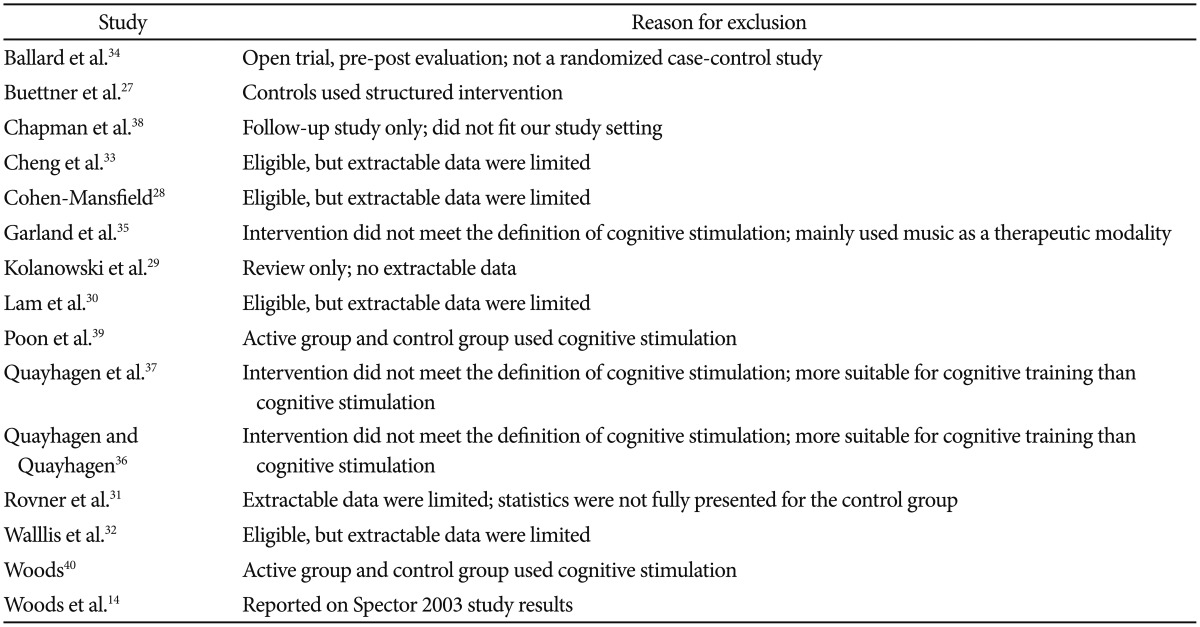
Cognitive Stimulation as a Therapeutic Modality for Dementia: A Meta-Analysis
Article information
Abstract
Objective
Although cognitive stimulation (CS) is one of the most popular non-pharmacological interventions for people with dementia, its efficacy is still debatable. We performed a meta-analysis of randomized controlled trials (RCTs) on the efficacy of CS in people with dementia.
Methods
Data sources were identified by searching PubMed, MEDLINE, Embase, psychINFO, and Cochrane Reviews Library. A total of 7,354 articles were identified, and of these, 30 RCTs were selected based on the selection criteria. Of these 30 RCTs, 14 were finally included in our meta-analysis [731 participants with dementia; 412 received CS (CS group) and 319 received usual care (control group)].
Results
We found that the people with dementia had a moderate benefit from CS. The mean difference between the CS and control groups was 2.21 [95% CI (0.93, 3.49), Z=3.38, p=0.00007] in the Alzheimer's Disease Assessment Scale-Cognition and 1.41 [95% CI (0.98, 1.84), Z=6.39, p<0.00001] in the Mini-Mental State Examination. CS also improved quality of life in people with dementia [95% CI (0.72, 3.38), Z=3.02, p=0.003].
Conclusion
CS is effective for improving cognition and quality of life in people with dementia; however, its effects were small to moderate.
INTRODUCTION
Dementia has become one of the most challenging global health problems. The number of people with dementia has been estimated to be 35.6 million, and the number has been predicted to double every 20 years and reach 115.4 million by 2050.1 In Korea, one of the most rapidly aging countries in the world, the number of people with dementia is expected to double every 17 years.2 People with dementia show gradual but progressive loss of cognition, and more than half of the people with dementia experience behavioral and psychological symptoms (BPSD).3 These people need supervision or support for their instrumental and basic activities of daily living (ADL). Caregivers of people with dementia experience physical, emotional, and financial burdens. The efficacy of cholinesterase inhibitors for dementia has been reported to be limited,456 and there is currently no cure for dementia. The importance of non-pharmacological interventions for people with dementia has increased.
Non-pharmacological interventions in combination with pharmacotherapies have been widely used in the management of people with dementia.78 Neural plasticity and capacity for cognitive-deficit compensation may underlie the efficacy of non-pharmacological interventions.9 Cognitive stimulation (CS) is one of the most popular non-pharmacological interventions for people with dementia.10 It is usually provided in a group and more flexible than cognitive training as it does not have to match specific therapeutic modalities. Several previous studies reported that CS may delay functional impairments1112 and improve quality of life (QoL)131415 in people with dementia. The Scottish Intercollegiate Guidelines Network (SIGN) recommends the application of CS to people with dementia in the UK.16 However, the therapeutic efficacy of CS in people with dementia has been in controversy because of the diversity of outcome measures and definition of CS employed in previous clinical trials. Several studies on CS were not clear with regard to the concepts of “training,” “stimulation,” and “rehabilitation.”
We performed a meta-analysis to investigate the effectiveness of CS on cognition, BPSD, mood, ADL, and QoL in people with dementia. In this meta-analysis, we defined CS as “an engagement in a range of activities and discussions aimed at general enhancement of cognitive and social functioning” proposed by Clare et al.111718
METHODS
We followed the meta-analysis guidelines corroborated by the National Evidence-based Health Care Collaborating Agency (NECA), Korea.19
Inclusion criteria of the studies
The study flow chart is presented in Figure 1. This meta-analysis focused on RCTs that provided relevant statistical information. Only studies published in English were considered for inclusion. The following criteria were considered: 1) participants had a diagnosis of dementia; 2) all levels of dementia severity that were indicated through group mean scores, range of scores, or individual scores using standardized scales, such as the Mini-Mental State Examination (MMSE)20 and Clinical Dementia Rating (CDR),21 were included; 3) data from family members or caregivers were not included; 4) age of the participants and type of dementia were not limited; and 5) the number of participants taking cognitive enhancers was mentioned if necessary.
We considered CS as “an engagement in various activities and discussions (usually in a group) aimed at general enhancement of cognitive and social functioning,” according to the definition proposed by Clare et al.11 We regarded “no treatment,” “usual care,” and “standard treatment” as controls. The term ‘no treatment’ was cognitive enhancer, clinic consultations, or contact with mental health team without any structured intervention that could be normally provided in usual treatment settings. There were no restrictions on the duration of interventions or the number of sessions. However, these values were noted.
The following variables were considered as outcome measures for the analyses: 1) the MMSE, Alzheimer's Disease Assessment Scale-Cognition (ADAS-Cog),22 Montreal Cognitive Assessment (MoCA),23 or other cognition assessment measures for cognitive impairment associated with global cognitive function; 2) self-reported function, clinically rated function, or caregiver-reported function measures for mood; 3) caregiver-reported function or clinically-rated function measures for behavioral and psychological symptoms; 4) self-reported function or caregiver-reported function measures for QoL, such as the QoL scale in Alzheimer's disease (QoL-AD)24; and 5) self-reported function or caregiver-reported function measures for ADL.
Search methods for the identification of studies
We searched the electronic medical databases PubMed, MEDLINE (1966 to April 2015), Embase (1980 to April 2015), psychINFO (1887 to April 2015), and Cochrane Reviews Library (1982 to April 2015) for all relevant English articles. Because of the ambiguity regarding interventions, we selected multiple keywords to improve sensitivity. First, medical subject heading (MeSH) terms were used for literature search, including CS, cognitive rehabilitation, cognitive training, cognitive rehabilitation program, cognitive therapy, nonpharmacological, memory training, exercise, physical activity, music therapy, art therapy, horticulture therapy, occupational therapy, validation therapy, reality orientation, and reminiscence. We then used the Boolean operation (OR) for sensitive search. The search strategies were as follows:
#1 Search (dementia) OR mild cognitive impairment
#2 Search ((((((((((((((((cognitive rehabilitation) OR (cognitive stimulation) OR cognitive training) OR cognitive rehabilitation program) OR cognitive therapy) OR nonpharmacological) OR memory training) OR exercise) OR physical activity) OR music therapy) OR art therapy) OR horticulture therapy) OR occupational therapy) OR validation therapy) OR reality orientation) OR reminiscence))))))))))))))))
#3 Search #1 and #2 filters: Clinical trial; Controlled clinical trial; Randomized controlled trial; Systematic reviews; Humans
To identify additional studies, reviews were hand-searched for both published and unpublished trials, and the authors were contacted if required.
Data collection and analysis
Primary selection was performed by reviewers independently based on the abstract. Then, secondary selection was performed by all researchers after full review. Regular consensus meetings were held to select studies according to the inclusion criteria. Studies were excluded if 1) the subjects were cognitively normal or had other neurological disorders that did not meet the criteria of dementia (DSM-IV-TR)25; 2) subjects were not human; 3) interventions were non-pharmacological methods that did not involve CS or were pharmacological methods; 4) the study was a duplicate publication; and 5) we could not find the original abstract or article, or we could not translate the study to English. The reviewers used a standardized abstracted form adapted from the Korean National Evidence-based Health Care Collaborating Agency (NECA) 19. All included studies were reviewed and graded according to the risk of bias checklist, using the Cochrane approach (risk of bias table) (Figure 2).
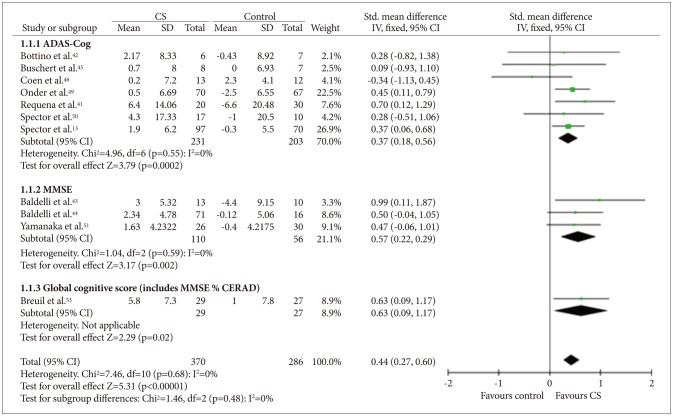
Cognitive stimulation versus no cognitive stimulation. Outcome: global cognition (overall). SD: standard deviation, Std. mean difference: standardized mean difference, IV, fixed: a fixed-effects meta-analysis with inverse variances weights, CI: confidence interval, df: degrees of freedom, tau2 and I2: heterogeneity values, Chi2: Chi-square test value, Z: Z-value as the overall effect, p: p-value.
The meta-analysis was performed using the software RevMan (Review Manager) Version 5.2. (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012). In most cases, summary effects were computed using a fixed-effects model, because the studies had similar settings and methodologies, and this approach provides more conservative result than a random-effects model. However, the random-effects model was used when statistical heterogeneity was proven by a Higgins' I-squared value of over 50% between studies.1926 We did not consider the long-term effects of interventions. Effect sizes were analyzed as standardized mean differences (SMDs), and Cohen's d was used with 95% confidence intervals (CIs) for comparisons between the active treatment (CS) and control (usual care) groups.
RESULTS
We identified a total of 7,354 articles in the literature search, and after deleting duplicate publications, 5,118 articles remained. After performing the processes of elimination, we identified 29 RCTs involving CS that met our inclusion criteria. Among these studies, 15 studies were excluded; seven studies did not have sufficient data for extraction,27282930313233 four studies employed different definition of CS,34353637 one study provided long-term follow-up data only,38 two studies compared the efficacy between two different types of CS,3940 and one study14 shared the same dataset with their previous study.13 For Requena 2006,41 the ‘no treatment’ group was excluded owing to non-randomization; therefore, comparisons were only performed for CS+ drug. Finally, 14 studies were included in this meta-analysis.1341424344454647484950515253 From these studies, we identified 731 participants [412 received CS and 319 used usual care (control)]. Further information on the included and excluded studies is presented in Table 1 and 2.
Efficacy for cognition
Data on cognition were available in 12 studies,121341424344454649505153 which included 370 participants who received CS and 286 who received usual care. The SMD between the CS and control groups was 0.44 [95% CI (0.27, 0.60)], and this value was statistically significant (fixed effect, Z=5.31, p<0.00001, I-square=0%) (Figure 2). As most of the studies used more than one outcome measure, analysis was performed on the most comprehensive measure. In seven studies that used the ADAS-Cog (Figure 3), the mean difference between the CS and control groups was 2.21 [95% CI (0.93, 3.49)], and this value was statistically significant (fixed effect, Z=3.38, p=0.00007, I-square=33%). In 11 studies that used the MMSE (Figure 4), the mean difference between the CS and control groups was 1.41 [95% CI (0.98, 1.84)], and this value was statistically significant (fixed effect, Z=6.39, p<0.00001, I-square=9%). However, we could not confirm the effectiveness of CS for specific cognitive domains as only one study reported on such effectiveness of CS.12 In that study, the improvement in language subscale (commands and spoken language items) was significantly better in the CS group than in the control group.

Cognitive stimulation versus no cognitive stimulation. Outcome: Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog). SD: standard deviation, IV, fixed: a fixed-effects meta-analysis with inverse variances weights, CI: confidence interval, df: degrees of freedom, tau2 and I2: heterogeneity values, Chi2: Chi-square test value, Z: Z-value as the overall effect, p: p-value.
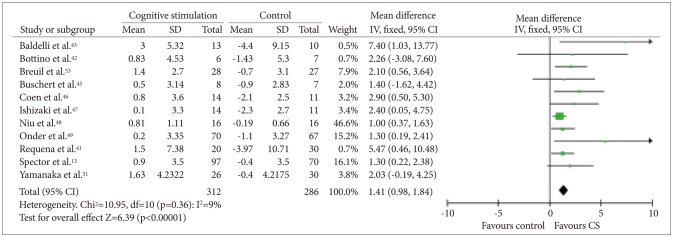
Cognitive stimulation versus no cognitive stimulation. Outcome: MMSE. SD: standard deviation, IV, fixed: a fixed-effects meta-analysis with inverse variances weights, CI: confidence interval, df: degrees of freedom, tau2 and I2: heterogeneity values, Chi2: Chi-square test value, Z: Z-value as the overall effect, p: p-value.
Behavior and psychological symptoms (BPSD)
Six studies134648495052 that included a total of 409 participants provided data on BPSD. Among these studies, three studies134650 used the Clifton Assessment Procedures for the Elderly (CAPE),54 two4849 used Neuropsychiatric Inventory (NPI),55 and one52 used the irritability and withdrawal subscales of the Multidimensional Observation Scale for Elderly Subjects (MOSES)56 (Figure 5). Quantitative analysis did not show any benefit of CS for the BPSD measures [random effect, SMD=0.32, 95% CI (-0.06, 0.70), Z=1.67, p=0.10].
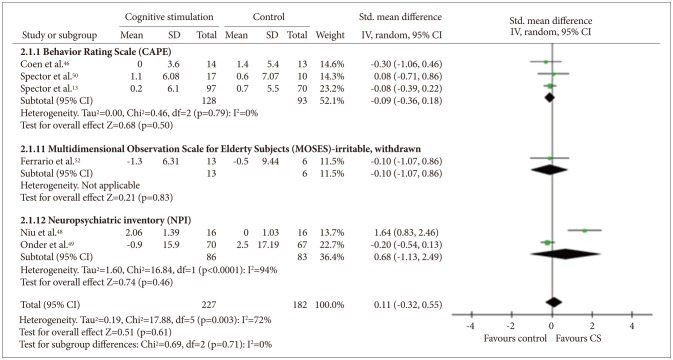
Cognitive stimulation versus no cognitive stimulation. Outcome: behavioral and psychological symptoms. SD: standard deviation, Std. mean difference: standardized mean difference, IV, random: a ramdom-effects meta-analysis with inverse variances weights, CI: confidence interval, df: degrees of freedom, tau2 and I2: heterogeneity values, Chi2: Chi-square test value, Z: Z-value as the overall effect, p: p-value.
Mood
Six studies414344454652 that included a total of 220 participants provided data on mood, and all described depression as a mood symptom. Among these studies, three studies414344 used the 30-item Geriatric Depression Scale (GDS-30),57 one 46 used the 15-item GDS (GDS-15),58 one 45 used the Montgomery-Asberg Depression Rating Scale (MADRS), and one52 used the depressed/anxious mood subscales of the MOSES (Figure 6). Quantitative analysis did not show any benefit of CS for the mood measures [fixed effect, SMD=0.20, 95% CI (-0.09, 0.50), Z=1.35, p=0.39].
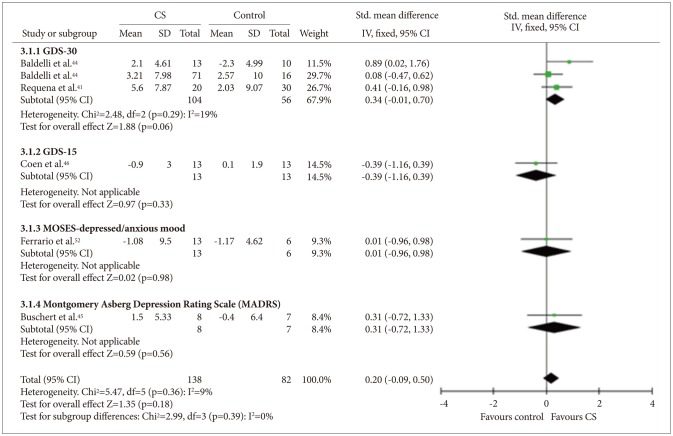
Cognitive stimulation versus no cognitive stimulation. Outcome: mood. SD: standard deviation, Std. mean difference: standardized mean difference, IV, fixed: a fixed-effects meta-analysis with inverse variances weights, CI: confidence interval, df: degrees of freedom, tau2 and I2: heterogeneity values, Chi2: Chi-square test value, Z: Z-value as the overall effect, p: p-value.
Quality of life (QoL)
Four studies45465051 that included a total of 265 participants provided data on QoL. All these studies used the QoL-AD (Figure 7). Quantitative analysis showed that CS was beneficial for QoL in people with dementia [fixed effect, SMD=2.05, 95% CI (0.72, 3.38), Z=3.02, p=0.003].
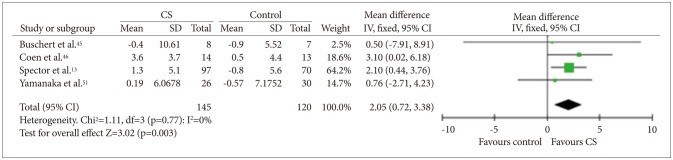
Cognitive stimulation versus no cognitive stimulation. Outcome: Quality of life. SD: standard deviation, IV, fixed: a fixed-effects meta-analysis with inverse variances weights, CI: confidence interval, df: degrees of freedom, tau2 and I2: heterogeneity values, Chi2: Chi-square test value, Z: Z-value as the overall effect, p: p-value.
ADL and instrumental ADL
Three studies434452 measured only basic ADL, and one study49 measured both basic and instrumental ADL (Figures 8 and 9). Quantitative analysis did not show any benefit of CS for basic ADL measures [fixed effect, SMD=0.19, 95% CI (-0.07, 0.45), Z=1.44, p=0.92]. Quantitative analysis for instrumental ADL was not possible because only one study assessed instrumental ADL and there was no significant difference in improvement between the study groups.
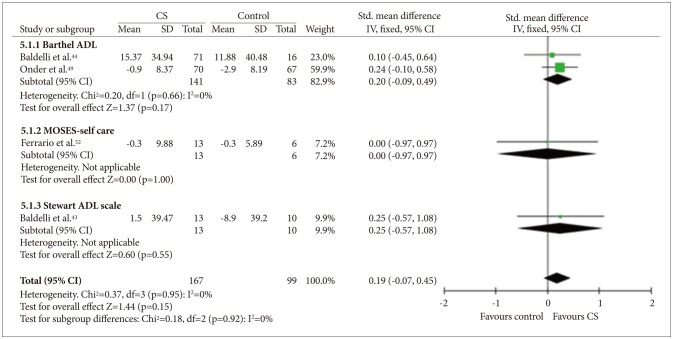
Cognitive stimulation versus no cognitive stimulation. Outcome: ADL. SD: standard deviation, Std. mean difference: standardized mean difference, IV, fixed: a fixed-effects meta-analysis with inverse variances weights, CI: confidence interval, df: degrees of freedom, tau2 and I2: heterogeneity values, Chi2: Chi-square test value, Z: Z-value as the overall effect, p: p-value.
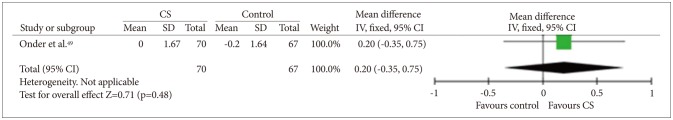
Cognitive stimulation versus no cognitive stimulation. Outcome: Instrumental ADL. SD: standard deviation, IV, fixed: a fixed-effects meta-analysis with inverse variances weights, CI: confidence interval, df: degrees of freedom, tau2 and I2: heterogeneity values, Chi2: Chi-square test value, Z: Z-value as the overall effect, p: p-value.
DISCUSSION
In this meta-analysis, CS was found to be effective for improving global cognition (1.80 points in the ADAS-Cog and 2.60 in the MMSE). The MMSE score has been reported to decline by 2–4 points annually in mild and moderate dementia patients.17 Although the improvement in global cognition with CS was not associated with a significant improvement in ADL, the findings portrayed an optimistic view of CS as a standard non-pharmacological therapy for dementia. Although it is yet not fully understood how CS preserves cognitive function in people with dementia, maintaining mental activities may protect against cognitive decline5960 by facilitating brain plasticity, proliferation and survival of hippocampal neurons,61 and the enlargement of the brain and/or cognitive reserve.62 In the present study, we could not perform a meta-analysis on the effectiveness of CS for each specific cognitive domain owing to the insufficient number of studies. However, in some previous studies,4750 CS was effective in improving commands and spoken language.
Maximizing QoL in people with dementia is one of the major goals in the management of dementia. As QoL in people with dementia is important to family caregivers and health service providers, as well as the people with dementia themselves, QoL is often included as an outcome measure in clinical trials on dementia.63 In the present study, although the effect size was small to moderate, we found that CS significantly improved the QoL-AD scores in people with dementia. Interventions that can enhance cognition have been reported to improve the sense of well-being and the QoL in people with dementia.30
In this study, CS was not beneficial for improving mood and BPSD, as in a previous Cochrane review.17 However, these results do not necessarily indicate that CS may not be helpful at all for improving mood and/or BPSD. The lack of efficacy of CS for mood, and BPSD in the present study may be attributed, at least in part, to the fact that people with dementia having severe mood symptoms or BPSD were excluded from most clinical trials on the efficacy of CS.53 In addition, some open-label studies have shown the efficacy of CS for mood and/or BPSD in people with dementia. For example, Ballard et al.34 reported that CS improved the Cohen-Mansfield Agitation Inventory score in people with dementia.
Current study has several limitations. First, most studies included in this meta-analysis had unclear risk of bias (Figure 10). We failed to find truly double-blinded studies in this analysis. The participants and staffs might have been aware of their treatment condition, which could lead to biased results. Second, there was broad heterogeneity in the study settings and the actual elements of the CS program. Third, the “sham” type of activities that were employed as control or placebo therapy had some components of CS according to the definition of Clare et al.11 Actually, unorthodox group activities have also been reported to be effective in people with dementia.174041 Fourth, the studies included in the present meta-analysis did not provide the efficacy of CS according to the type of dementia. The efficacy of CS may not be uniform across different types of dementia. Furthermore, the efficacy of CS has never been studied in rare types of dementia. Fifth, the analyses were insufficient to prove the quantitative therapeutic correlation of CS. Some studies in this analysis did not clearly present the duration and frequency of the intervention. Further research is warranted on the relationship between the intervention and the dose effect.
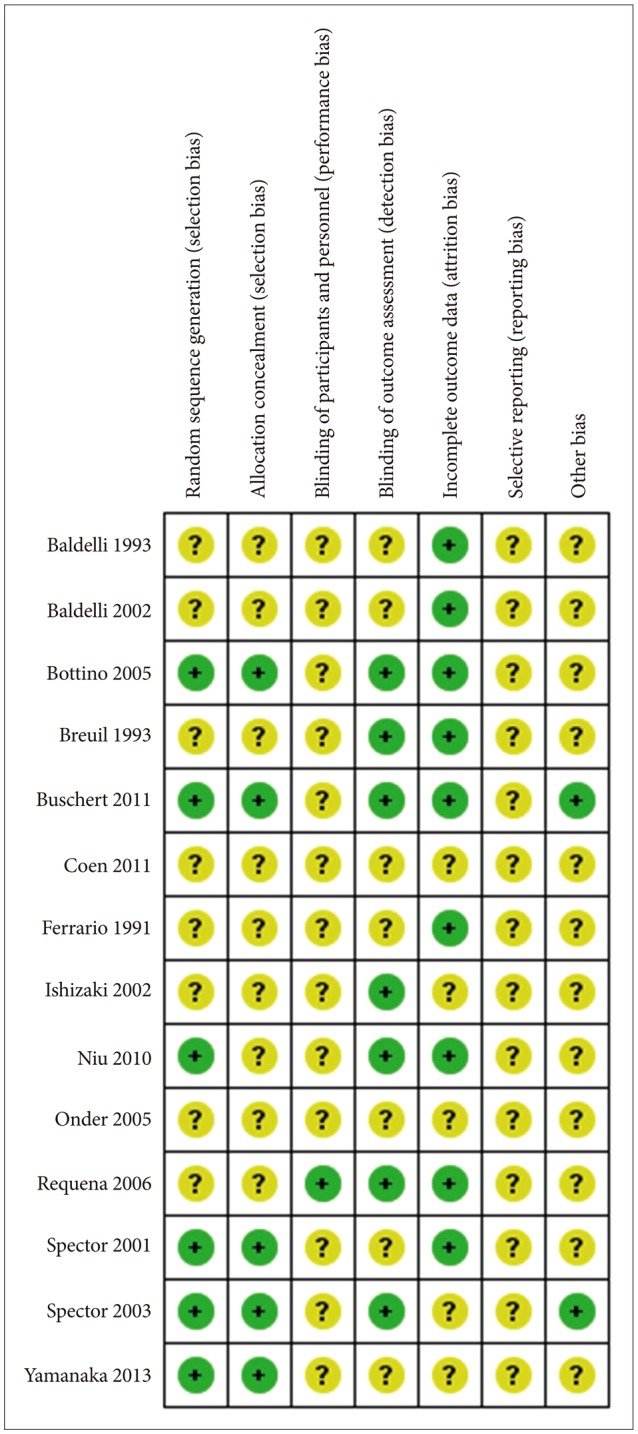
Risk of bias summary: Review of authors' judgments about each risk of bias item for each included study.
In conclusion, CS is more effective than usual activities for improving global cognition and QoL in people with dementia.
Acknowledgments
This study was supported by grants from the Korean Health Technology R&D Project, Ministry for Health, Welfare, and Family Affairs, Republic of Korea [grant number HI09C1379 (A092077)] and from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (grant number HI12C2018).