Genetic Polymorphism of 1019C/G (rs6295) Promoter of Serotonin 1A Receptor and Catechol-O-Methyltransferase in Panic Disorder
Article information
Abstract
Objective
Family and twin studies have suggested genetic liability for panic disorder (PD) and therefore we sought to determine the role of noradrenergic and serotonergic candidate genes for susceptibility for PD in a Japanese population.
Methods
In this age- and gender-matched case-control study involving 119 PD patients and 119 healthy controls, we examined the genotype distributions and allele frequencies of the serotonin transporter gene linked polymorphic region (5-HTTLPR), −1019C/G (rs6295) promoter polymorphism of the serotonin receptor 1A (5-HT1A), and catechol-O-methyltransferase (COMT) gene polymorphism (rs4680) and their association with PD.
Results
No significant differences were evident in the allele frequencies or genotype distributions of the COMT (rs4680), 5-HTTLPR polymorphisms or the −1019C/G (rs6295) promoter polymorphism of 5-HT1A between PD patients and controls. Although there were no significant associations of these polymorphisms with in subgroups of PD patients differentiated by gender or in subgroup comorbid with agoraphobia (AP), significant difference was observed in genotype distributions of the −1019C/G (rs6295) promoter polymorphism of 5-HT1A between PD patients without AP and controls (p=0.047).
Conclusion
In this association study, the 1019C/G (rs6295) promoter polymorphism of the 5-HT1A receptor G/G genotype was associated with PD without AP in a Japanese population.
INTRODUCTION
Panic disorder (PD) is characterized by the spontaneous, unexpected, and repetitive occurrence of panic attacks.1 These attacks are relatively short-lived episodes (usually <1 hour) of intense anxiety or fear accompanied by somatic symptoms such as palpitations and tachypnea.1 Patients with PD commonly experience anticipatory anxiety about the recurrence of panic attacks. They may also have agoraphobia (AP), the fear of being alone in public places, especially those from which a rapid exit would be difficult.1 In the a recent epidemiological survey, lifetime prevalence estimates were reported to be 22.7% for isolated panic attacks without AP, 0.8% for panic attacks with AP without PD, 3.7% for PD without AP, and 1.1% for PD with AP.2 A sex difference in PD has been established, with women showing a higher prevalence (5.6%) than men (1%).3 Family and twin studies have suggested a genetic liability for PD.456 Heritability has been estimated at around 48% in a meta-analysis study.7
Charney et al.8 suggested that sensitivity to augmented noradrenergic function is increased in PD and AP. The plasma-free level of the norepinephrine metabolite 3-methoxy-4-hydroxyphenylglycol (MHPG) is correlated with increases in ratings of anxiety in PD with AP.9 This might indicate either higher production or faster degradation of norepinephrine in PD.10 Catechol-O-methyltransferase (COMT) is one of the major methylation enzymes metabolizing catecholamines, such as norepinephrine. The COMT gene maps to chromosome 22q11.2 and contains a 472G/A single-nucleotide polymorphism (rs4680) causing an amino acid substitution from valine to methionine in codon 158 (val158met) of the membrane-bound form of the enzyme [codon 108 (val108met) of the soluble form].11 The valine allele (472G) is associated with 40% higher COMT activity compared with the methionine allele (472A).12 Lachman et al.11 reported that homozygosity of COMT158met leads to a 3 to 4-fold reduction in enzymatic activity, compared with the homozygotes for COMT158val. A meta-analysis provides tentative support for the COMT val158met polymorphism as a possible risk factor for PD, with differential effects seen in Caucasian and Asian populations, and suggests a female-specific effect.13 Moreover, Freitag et al.10 reported that the combination of the COMT gene (472GA) and −1019C/G (rs6295) promoter gene is associated with PD, and particularly with PD without AP in a Caucasian population.
The serotonin (5-HT) transporter (5-HTT) gene has been extensively screened for polymorphic variants. The 5-HTT gene-linked polymorphic region (5-HTTLPR), located in the promoter region, has been identified as a functional polymorphism. The polymorphism consists of a 44-base pair (bp) insertion or deletion involving repeat elements 6 to 8.14 In vitro, the basal activity of the long (L) variant was found to be more than twice that of the short (S) one in 5-HTT mRNA synthesis and 5-HTT expression.14 These two different transcriptional efficiencies suggest that 5-HTT gene transcription is modulated by 5-HTTLPR genetic variants.14 However, association studies have reported no significant difference in 5-HTTLPR allele frequencies between patients with PD and controls.15161718 A systematic review and meta-analysis also showed no evidence to support an association between 5-HTTLPR and PD.19
The −1019C/G (rs6295) promoter polymorphism of the serotonin receptor 1A (5-HT1A) has been found to be associated with major depression and anxiety.20 In addition, Rothe et al.21 reported a possible association between the −1019C/G 5-HT1A gene promoter polymorphism and PD with or without AP in a case-control study. While they found no significant evidence of allelic association with PD overall, they did find a significant association with the G allele and PD with AP. Recent positron emission tomography (PET) studies in humans have reported reduced 5-HT1A receptor binding in patients with PD. Neumeister et al.22 reported the potential was lower in the anterior cingulate, posterior cingulate, and raphe nucleus in PD patients, while David et al.23 reported it to be lower in all brain regions in healthy subjects with 5-HTTLPR short genotypes (S/S and S/L) compared with those with L/L homozygotes, although they found no significant effects of the 5-HT1A receptor genotype on 5-HT1A receptor binding.
Given that these findings suggest functional participation of both the noradrenergic and serotonergic gene polymorphisms in PD, we conducted the present age- and gender-matched case-control study to investigate interactions between the relevant genes [i.e., COMT gene (rs4680; 472GA), 5-HTTLPR gene, and −1019C/G (rs6295) promoter gene] and PD in a Japanese population.
METHODS
Subjects
One hundred nineteen unrelated Japanese patients who met the criteria for diagnosis of PD according to Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR) were included in the study (Table 1). All PD patients [43 men, 76 women; age, 35.45±9.50 years (mean± SD); age range, 18−58 years] were outpatients at Dokkyo Medical University Hospital or Sakura La Mental Clinic, Japan. The following exclusion criteria were applied: 1) axis I diagnosis other than PD; 2) axis II diagnosis; 3) severe physical illness or major laboratory test abnormalities; 4) suicide risk; or 5) history of substance abuse.
The control group consisted of 119 unrelated Japanese individuals matched with the patient group by gender and age (Table 1). They were recruited from personnel at Dokkyo Medical University Hospital and Sakura La Mental Clinic. All were healthy and did not manifest any psychiatric problems in unstructured interviews conducted by psychiatrists.
The ethics committees of Dokkyo Medical University Hospital and Sakura La Mental Clinic approved the study. Written informed consent was obtained from all PD and control subjects following an explanation of the study procedure.
Genotyping
DNA was isolated from blood cell fractions using the QIAamp Blood Kit (Qiagen Inc., Chatsworth, CA, USA). The 5-HTTLPR genotypes (L and S alleles) were determined by polymerase chain reaction (PCR) as described by Lesch et al.24 and Heils et al.14 with minor modifications. Oligonucleotide primers flanking the 5-HTTLPR and corresponding to the nucleotide positions -1416 to -1397 (LPR5; 5′-GGCGTTGCC GCTCT GAATTGC) and -910 to -889 (LPR3; 5′-GAGGGACT GAGCTGGACAACCCAC) of the 5-HTT gene regulatory region were used to generate a 484/528-bp fragment. PCR amplification was performed in a final volume of 12.5 µL containing 20 ng genomic DNA, 0.8 mM dNTP mixture, 0.05 µg sense and antisense primers (LPR5 and LPR3), 1×PCR buffer, 1.5 mM MgCl2, 5% dimethyl sulfoxide, and 0.5 U AmpliTaq DNA polymerase® (Applied Biosystems Japan Ltd., Tokyo, Japan). Annealing was performed at 60℃ for 30 s, extension at 72℃ for 1 min, and denaturation at 94℃ for 30 s for 35 cycles.
The rs6295 single nucleotide polymorphism (SNP) in the 5-HT1A receptor gene and rs4680 SNP in COMT were analyzed using TaqMan SNP Genotyping Assays® (Assay ID: C_11904666_10 and C_25746809_50, respectively; Applied Biosystems, Foster City, CA). PCR amplification was performed in a total volume of 10 µL containing 1×LA PCR buffer, 2.5 mM MgCl2, 0.25 mM of each dNTP, 1×TaqMan probe® (Applied Biosystems), 20 ng genomic DNA, and 0.5 U TaKaRa LA Taq® (Takara Bio Inc., Shiga, Japan) in a TP800 thermal cycler Dice real time system (Takara Bio Inc.,). FAM-MBG dyes were detected as the major allele and VIC-MBG dyes as the minor allele. The reaction conditions were as follows: 95℃ for 1 min 45 s, 40 cycles of 92℃ for 15 s, and 60℃ for 1 min.
Statistical analysis
The individual genotype distributions and allele frequencies for the PD patients and controls were compared using the chi-square test or two-sided Fisher's exact test. Hardy-Weinberg equilibrium was assessed with the chi-square test. These statistical analyses were performed with SNPAlyze ver.8.0 standard (http://www.dynacom.co.jp/product_service/packages/snpalyze/). The interaction between the associated polymorphisms was examined by multilogistic regression analysis. This statistical analysis was calculated with SPSS statistics version 19.0 (Japan IBM, Tokyo, Japan).
RESULTS
Allele frequencies and genotype distributions
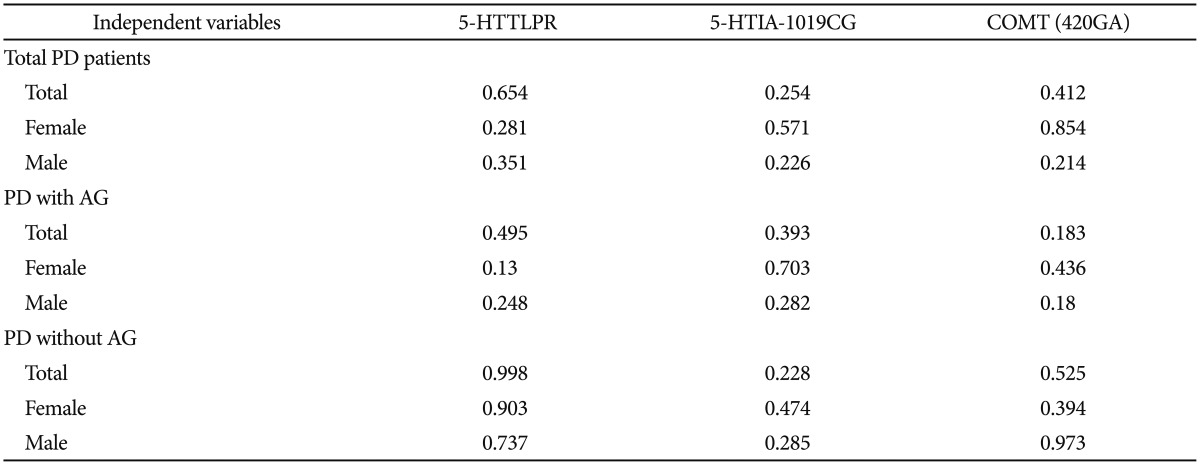
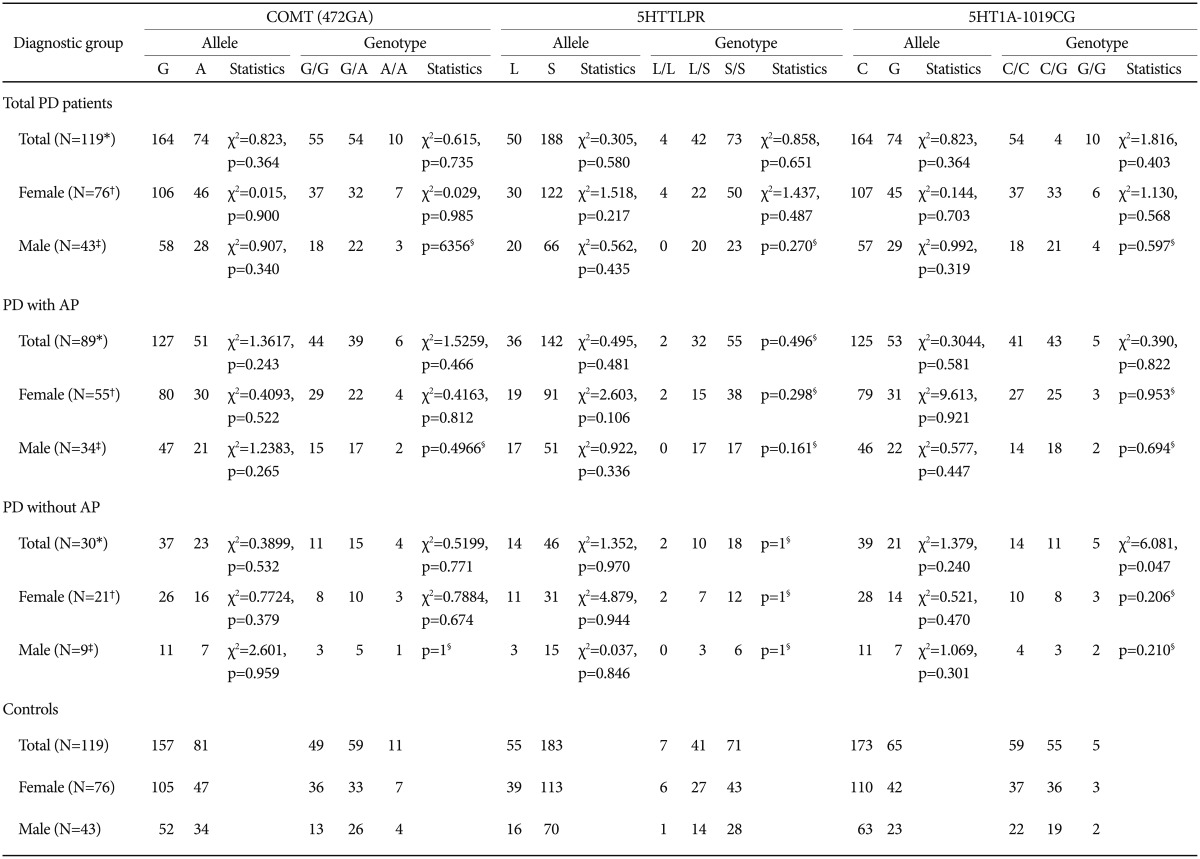
Results for the 5-HTTLPR, −1019C/G (rs6295) promoter, and COMT (rs4680) genes are shown in Table 2. In the case of both the COMT (rs4680) polymorphism and the 5-HTTLPR polymorphism, the genotype frequencies did not deviate significantly from Hardy-Weinberg equilibrium. No significant differences in the allele frequencies or genotype distributions were found between any of the groups examined; that is, no differences were seen between all (female+male) PD patients and all (female+male) controls, female PD patients and female controls, male PD patients and male controls, female PD patients with AP and female controls, male PD patients with AP and male controls, female PD patients without AP and female controls, and male PD patients without AP and male controls.
Allele frequencies and genotype distributions of COMT (rs4680), the 5-HTTLPR, and 5HT1A −1019C/G (rs6295) polymorphism
In regard to the 5HT1A −1019C/G (rs6295) polymorphism, genotype frequencies were also in Hardy-Weinberg equilibrium. As shown in Table 2, there were no significant differences in the allele frequencies and genotype distributions of the −1019C/G (rs6295) polymorphism between all (female+male) PD patients and all (female+male) controls; however, a significant association was found between the G allele and PD without AP (n=30; χ2=6.0814, p=0.0478) (Table 2). In the recessive model of PD without AP, a significantly higher frequency of the G/G genotype was observed in PD without AP than in controls (16.7% vs. 4.2%, χ2=5.946, p=0.0148).
Interactions between the 5-HTTLPR, 5HT1A −1019C/G (rs6295), and COMT (rs4680) polymorphisms
The results are shown in Table 3. Multilogistic regression analysis was performed to analyze association of the genetic polymorphisms of 5-HTTLPR, 5HT1A −1019C/G (rs6295), and COMT (rs4680) with PD, where subgroups differentiated by gender, with AP, and without AP served as the dependent variables and the 5-HTTLPR, −1019C/G (rs6295), and COMT (rs4680) polymorphisms served as the independent variables. No significant associations were found between any of the dependent and independent variables.
DISCUSSION
The COMT rs4680 G-allele was first reported to be associated with PD in female Caucasian patients in 2004.25 A meta-analysis a few years later provided tentative support that the allele is a risk factor for PD in this specific patient subgroup, and also indicated that the A-allele is associated with PD in female Asian patients.13 It is known that the COMT rs4680 G-allele leads to faster extraneuronal inactivation of monoaminergic neurotransmitters.11 A local reduction in norepinephrine availability at the alpha-1 heteroreceptors on serotonergic raphe neurons might lead to a reduction in the firing rate of these neurons.26 In PD, reduced serotonergic neurotransmission caused by elevated COMT activity due to the rs4680 G-allele or by enhanced 5-HT1A autoreceptor/reduced 5-HT1A heteroreceptor expression due to the −1019C/G G allele might be the pathogenetic mechanism responsible for the condition.10
The −1019C/G 5-HT1A gene promoter polymorphism was previously found to impair transcriptional repression of the 5-HT1A receptor gene by means of trans-acting nuclear-deformed epidermal autoregulatory factor (DEAF)-1-related protein (NUDR).27 The C-allele was part of a 26-bp imperfect palindrome that bound NUDR, whereas the G-allele abolished repression by NUDR. More importantly, stable expression of NUDR greatly reduced the expression of 5-HT1A receptors, indicating that NUDR negatively regulates both 5-HT1A gene transcription and receptor expression in the dorsal raphe nucleus.27 As suggested by Le Francois et al.,20 it is possible that the G-allele and G/G genotype are associated with increased expression of the 5-HT1A autoreceptor in 5-HT neurons. The present study and previous association studies concur in the finding that the 5-HTTLPR polymorphism does not affect PD.
In addition, we found no significant difference between PD and controls in allele frequency and genotype distribution of the 5-HTTLPR, or COMT (rs4680) polymorphisms, however, we found significant difference in 5HT1A −1019C/G genotype distribution between PD without AP and controls.
It is well established that there is a difference in allele frequency in the 5-HTTLPR polymorphism between Caucasian and Asian populations. Likewise, ethnic differences exist in the allele frequency and genotype distribution of the COMT (472GA) polymorphism between Caucasian and Japanese populations (Table 4). The allele frequency of the COMT 472GA (rs4680) G-allele has been reported in healthy Caucasian subjects as 0.415 (93/224),10 0.413 (95/230),25 and 0.519 (135/260)28 and in healthy Japanese subjects as 0.715 (199/276)29 and 0.660 (present study). We assume that the COMT 472GA (rs4680) G-allele did not affect PD in the Japanese subjects in the present study because of the ethnicity-related difference in allele frequency.
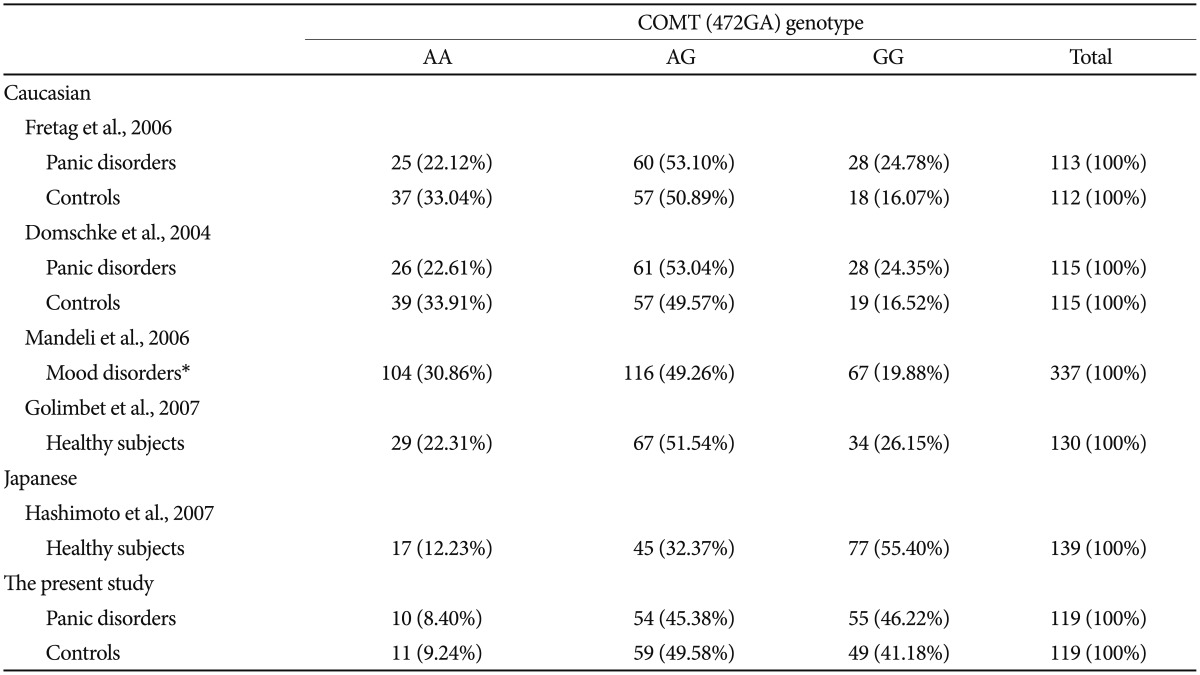
Ethnic differences in genotype distribution of the COMT (472GA) polymorphism between Caucasian and Japanese populations
The prevalence of PD in women is more than twice that in men.3 There are several genetic studies of this female-specific association in PD with respect not only to COMT rs46801325 variants, but also to the monoamine oxidase A (MAOA) variation that exists in the form of a 30-bp variable number of tandem repeats (VNTR) polymorphism in the MAOA promoter region.30 On the other hand, angiotensin-converting enzyme (ACE) localized on chromosome 17q23, a functional insertion deletion (I/D) polymorphism I allele, may be associated with PD in a potentially male-specific manner.3132 ACE degrades substance P, which has been implicated in anxiety-related behavior.31 ACE has been also suggested as a potential risk factor in the pathogenesis of panic attacks,31 although the ACE gene was not found to be associated with PD in a Japanese population.33
The present study found no gender-specific gene effects, despite gender-specific genes related to PD being reported in a Caucasian population. However, we did find some similar results to those for a Caucasian population.10 In our Japanese population, the G/G genotype of −1019C/G (rs6295) promoter polymorphism of 5-HT1A was associated with PD without AP, and the association of the G-allele of the −1019C/G (rs6295) promoter polymorphism with PD has been reported in Caucasian subjects.10 The findings suggest that the G/G genotype of −1019C/G (rs6295) promoter polymorphism of the 5-HT1A might affect PD without AP in both Japanese and Caucasian populations.
There are some limitations to this study. First, the sample size was relatively small and is not sufficient to reach any robust conclusions. Second, other serotonergic and noradrenergic gene polymorphisms were not investigated. For example, the MAOA 30-bp VNTR polymorphism might be a risk factor in female PD patients not only among Caucasians, but also among Japanese. Also, the 5-HT2A 102T/C polymorphism might be associated with PD, given that a positive association has been reported in an association study in a Japanese population.34
Acknowledgments
This study was funded by a Grant-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Research (#25461739) and Seki Minato Memorial Awards to Kazutaka Shimoda. The funding sources played no role in the study design; collection, analysis, or interpretation of data; writing of the report; or decision to submit the paper for publication.
Notes
Conflict of interest: Kazutaka Shimoda has received research support from Daiichi Sankyo Co., Dainippon Sumitomo Pharma Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan, K.K., GlaxoSmithKlein K.K., Meiji Seika Pharma Co., Ltd., Novartis Pharma K.K., Otsuka Pharmaceutical Co., Ltd., Pfizer Inc., Shionogi & Co., Ltd., Takeda Pharmaceutical Co., Ltd., Tsumura & Co., and Yoshitomi Pharmaceutical Industries, Ltd., and honoraria from Asahi Kasei Pharma Corporation, Astellas Pharma Inc., Daiichi Sankyo Co., Dainippon Sumitomo Pharma Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan, K.K., GlaxoSmithKlein K.K., Janssen Pharmaceutical K.K, Kowa Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Mitsubishi Tanabe Pharma Corporation, Ltd., MSD K.K., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., Pfizer Inc., Shionogi & Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Tsumra & Co., from the year of 2012 to 2014. The authors other than Kazutaka Shimoda declare no biomedical or financial interests or potential conflicts of interest directly relevant to the content of the present study.