Failure to Detect Borna Disease Virus Antibody and RNA from Peripheral Blood Mononuclear Cells of Psychiatric Patients
Article information
Abstract
Objective
Borna disease virus (BDV) is a highly neurotropic agent causing various neuropsychiatric symptoms in animals. Over the past two decades, it has been suggested that BDV might be associated with human psychiatric diseases. We aimed to investigate whether BDV is associated with psychiatric patients in Korea.
Methods
We recruited 60 normal controls and 198 psychiatric patients (98 patients with depressive disorder, 60 with schizophrenia, and 40 with bipolar disorder). We used an indirect immunofluorescence antibody (IFA) test for the BDV antibody and a real-time reverse transcriptase polymerase chain reaction (rRT-PCR) assay for p24 and p40 RNA from peripheral blood mononuclear cells (PBMCs).
Results
Neither the BDV antibody nor p24, p40 RNA was detected in controls and patients groups.
Conclusion
Our results suggest that BDV might not be associated with psychiatric patients in Korea.
Introduction
It has been suggested that viruses may cause various psychiatric diseases such as schizophrenia and mood disorders.1 Borna disease virus (BDV) is one of the possible causative agents associated with psychiatric diseases. BDV is a highly neurotropic RNA virus with an enveloped, nonsegmented, negative stranded RNA genome.2-4 BDV has been known to naturally infect several animal species such as cattle, cats, horses, and sheep.5-8
Animals infected with BDV show various neurobehavioral symptoms, such as hyperactivity, stereotyped behavior, anxiety, and abnormal social behaviors reminiscent of symptoms observed in human psychiatric diseases.9-11 BDV mainly infects the limbic system and cerebellum, which play an important role in the psychiatric disease.12-14 Recent studies have further demonstrated evidence that BDV causes disturbances in the central nervous system.15-17
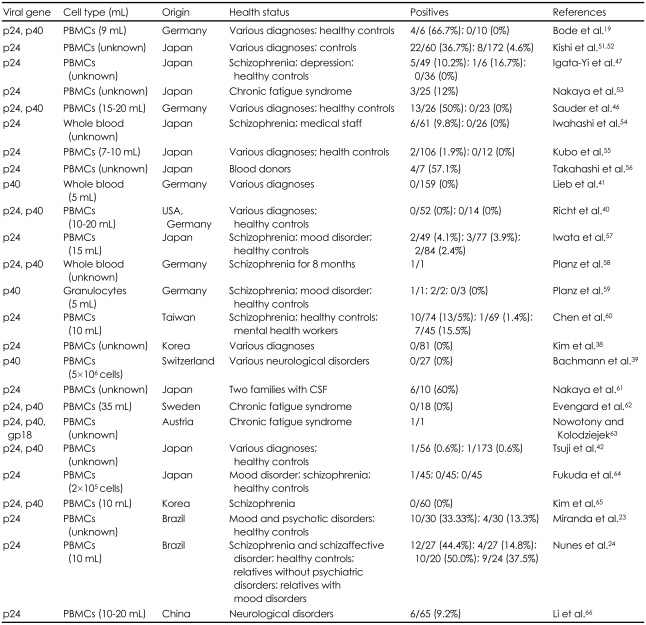
Based on those findings, several studies have been carried out to investigate whether BDV is associated with psychiatric diseases. Initially, Rott et al.18 detected antibodies against BDV mainly in mood disorder patients. With the knowledge of the sequence and genomic organization of BDV, Bode et al.19 first detected BDV RNA by reverse transcriptase polymerase chain reaction (RT-PCR) in various psychiatric patients. Other investigators have revealed the possible relationship between BDV and human psychiatric diseases in various regions such as Europe,20-22 Brazil,23,24 and Japan.13,25,26 However, due to the lack of reliable diagnostic tools for BDV detection, subsequent studies could not replicate BDV-positive results (Table 1), and it remains unclear whether BDV is associated with human psychiatric diseases.27
Recently, real time RT-PCR (rRT-PCR) has been proven to be an effective and convenient method in viral gene detection.28,29 rRT-PCR has the advantage of avoiding the contamination problem during the procedure, which is a drawback of nested RT-PCR.30 Nested RT-PCR comprises two consecutive rounds of PCR amplification to improve sensitivity. Generally, those two PCR amplification process is performed in two tubes, which requires manual handling of amplicons. Also, to detect and prevent the contamination of complementary DNA (cDNA), both positive and negative controls are required in each PCR rounds. Hence, the cross-contamination would occur between primary and secondary PCR. After the secondary PCR is finished, it is needed to transfer the nested PCR products to the agarose gel electrophoresis to detect the products. This process also increases the risk of contamination. However, in the case of rRT-PCR, the risk of contamination is low because both the PCR and detection of the products are performed in a sealed system without handling of amplicons. Several studies have established the sensitivity and specificity of rRT-PCR for the detection of BDV genes.31,32 Hence, we used rRT-PCR to investigate BDV infection in psychiatric patients. To our knowledge, it is the first study to examine BDV RNA in psychiatric patients by rRT-PCR. Considering some evidence indicating discrepancies between serologic studies and rRT-PCR results,33 we used both an indirect immunofluorescence antibody (IFA) test and rRT-PCR to compare the results of the two methods. This study investigated BDV RNA and BDV antibody using rRT-PCR and indirect IFA test from peripheral blood mononuclear cells of psychiatric patients in Korea.
Methods
Subjects
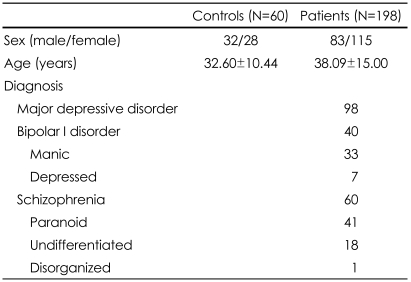
During January 2004 and December 2007, 198 psychiatric patients and 60 normal controls were recruited. All the patients were newly admitted in closed wards of the Department of Psychiatry, Ansan Hospital. Of the 198 patients, 98 patients had major depressive disorder, 60 had schizophrenia, and 40 had bipolar disorder. All the patients were interviewed by structured diagnostic criteria categorized according to the criteria of the fourth edition of the American Psychiatric Association.34 All the patients had active symptoms at the time of enrollment. Sixty normal controls were randomly selected among healthy individuals visiting the same hospital for regular health screens. All the patients and controls gave informed consent after a complete description of the study. The study protocol was approved by the Ethics Committee of Korea University.
Preparation of peripheral blood mononuclear cells
A sample of fasting blood (20 mL) was drawn from each of the 198 patients and 60 controls. Preparation of peripheral blood mononuclear cells (PBMCs) were isolated from anticoagulant-treated blood by Ficoll-hypaque gradient centrifugation. Total RNA was extracted from PBMCs using RNAzol (Gibco/BRL, Gaithersburg, MD, USA).
Serological detection of Borna disease virus infection with an indirect immunofluorescent antibody test
An indirect IFA test was conducted according to the method previously described.18 The Madine Darby canine kidney (MDCK) cell line and MDCK cell line persistently infected with BDV (MDCK-BDV)35 cultured for five days with Dulbecco's Modified Eagle Medium (DMEM)(Gibco/BRL, Germany) supplemented with 10% fetal bovine serum (FBS)(Gibco/BRL, Germany) were trypsinized, suspended in phosphate buffered saline (PBS), spotted onto Teflon-coated 10-well slides, and air-dried in room temperature. Cells on the 10-well antigen slide were fixed with anhydrous acetone at -20℃ for 7 minutes and dried. Human and horse serum samples diluted in PBS as 1 : 32 were treated to the MDCK cell and MDCK-BDV cell on the antigen slide, and the slide was incubated in a humidity chamber at 37℃ for 30 minutes. After three washes with PBS, Fluorescein isothiocyanate (FITC) conjugated anti-human immunoglobulin goat IgG (MP Biomedicals Inc., USA) was added to each well, and the slide was incubated in the humidity chamber at 37℃ for 30 minutes. The slides were mounted with glycine-buffered glycerol under cover slips and examined for a characteristic cytoplasmic fluorescent pattern with a fluorescence microscope.
Genetic detection of viral genomic RNA with real-time reverse transcription-polymerase chain reaction
Real-time quantitative RT-PCR for nucleoprotein (p40) and phosphoprotein (p24) genes of BDV was performed to detect the RNA genome of BDV in PBMCs. TaqMan probes were labeled with 6-carboxy fluorescein (FAM) as the 5' fluorescent reporter and tetramethylrhodamine (TAMRA) as the 3' quencher. The primers and probes were made as follows, in accordance with the previous report:36
p40 forward primer: 5'-TTTCATACAGTAACGCCCAGCC-3'
p40 reward primer: 5'-GGCGTCGACAGGTAAGATTCA-3'
p40 probe: 5'-FAM-TGAACAAACGCAGCGTGCAGTCCTTAMRA-3'
p24 forward primer: 5'-ATGCATTGACCCAACCGGTA-3'
p24 reward primer: 5'-ATCATTCGATAGCTGCTCCCTTC-3'
p24 probe: 5'-FAM-AGAACCCCTCCATGATCTCAGACCCAGA-TAMRA-3'
For the construction of the quantitative standard, the p40 and p24 genes were amplified by RT-PCR and cloned to pGEM-T Easy vector system (Promega, USA). PCR primers were made as follows:
p40-BV259F: 5'-TTCATACAGTAACGCCCAGC-3'
p40-BV829R: 5'-GCAACTACGGGGATTGTAAGGG-3'
p40-OSX458: 5'-ATGCATTGACCCAACCAGTA-3'
p24-OSX459: 5'-GTCCCATTCATCCGTTGTC-3'
Cloned p40 and p24 genes were transcribed to RNA using the MEGAscript T7 transcription kit (Ambion Inc., USA) in accordance with the manufacturer's instructions. A reverse transcription reaction was performed on the final 1×1010 copies of cDNA per 1 µL using the p40-F and p24-F primers. The cDNA mixtures were diluted by the 10-fold dilution method until 1×103 copies of cDNA per 1 µL were obtained. The real-time PCR reaction mixture consisted of the Taqman Universal PCR Master Mix (Applied Biosystems, USA), as recommended in the manufacturer's instructions. The real-time PCR reaction mixtures were incubated for 10 minutes at 95℃, and 40 cycles of amplification were performed using the ABI PRISM 7000 Sequence Detection System (Applied Biosystems, USA), each cycle consisting of a denaturation step (15 seconds at 95℃) and an annealing-elongation step (1 minute at 60℃). The threshold cycle (Ct) values, or the number of cycles for fluorescence to reach clearly detectable levels, a value greater than 40 was regarded as a negative result (Table 2).
Statistical analysis
To analyze the demographic data, a two-tailed t-test was used for continuous covariates. For discrete covariates, a chi-square test was used. The null hypothesis was rejected at p<0.05. The statistical package used for the analysis was Statistical Package for Social Science (SPSS; SPSS Inc, Chicago, IL, USA) 11.01.
Results
Demographic data
Normal controls and psychiatric patients were compared on age and sex (Table 3). There was no significant difference in the male/female ratio between the controls and patients groups. However, the controls were significantly older than the patients (p=0.009).
Immunofluorescence antibody and real-time reverse transcriptase polymerase chain reaction
p24 and p40 RNA were not detected by rRT-PCR in the controls and patients. In addition, the BDV antibody was not detected by the IFA in either group.
Discussion
In this study, we failed to detect the BDV antibody or RNA from peripheral blood mononuclear cells (PBMC) in psychiatric patients. Due to no evidence of BDV infection, we could not compare the results of the IFA and rRT-PCR. Including our previous studies,37,38 there have been several studies suggesting a dissociation between BDV and human psychiatric diseases.33,39-42
There are several possible reasons for these controversial results. First, positive results might have been induced by laboratory artifacts such as contamination. As mentioned before, nested RT-PCR is prone to contamination during procedures. It has been suggested that BDV sequences found in human samples are very similar to the laboratory strains.43,44 Recently, in a meta-analytic study, Dürrwald et al.45 suggested that all the studies with BDV-positive results by nested RT-PCR might have used contaminated samples. The advantage of our study is that we used rRT-PCR which is the preferred methods to prevent cross-contamination. Because rRT-PCR could also have the possible risk of contamination, we specially paid attention to keep all the equipments cleaned and decontaminated. However, several studies have demonstrated that RT-PCR positive results were more frequent in patients than in controls.46,47 The findings suggest that contamination cannot fully explain the discrepancies in conflict RT-PCR results. With regard to the seroepidemiological study, the low avidity of human antibodies against BDV antigens compared to the high avidity of infected animal antibodies has raised the possibility that seroprevalence of BDV in humans might be the result of cross-reactivity.48 Second, the negative results might have been derived from the area in which the BDV infection of the animal is not endemic. The fact that we have consistently failed to demonstrate BDV infection in Korean psychiatric patients supports this notion.37,38 However, the conflicting results regarding BDV infection cannot be attributed only to geographic factors. It has been reported that BDV is not associated with psychiatric patients in Kyushu island in Japan, a known endemic area of BDV infection.8,42,49 However, there have been two BDV-positive reports in Brazil, which has not been known to be an endemic BDV infection area.23,24 Third, the pathogenesis and symptoms of BDV infection in humans would be different from the infection in animals. Recently, Matsunage et al.50 have suggested that human infection of BDV would be asymptomatic and non-pathogenic.
In conclusion, this study supports our previous results that BDV is not associated with psychiatric patients in Korea. However, it remains controversial whether BDV infection is associated with human psychiatric diseases. To clarify the presence of BDV in psychiatric patients, subsequent studies with novel diagnostic tools are needed.
Acknowledgments
Funding for this study was provided by a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (A040042).