Gender-Specific Association of the Brain-Derived Neurotrophic Factor Gene with Attention-Deficit/Hyperactivity Disorder
Article information
Abstract
Objective
Attention-deficit/hyperactivity disorder (ADHD) is a complex neurodevelopmental disorder with a strong genetic component. Brain-derived neurotrophic factor (BDNF), which participates in the differentiation and survival of dopaminergic and noradrenergic neurons, could play a role in ADHD development. We aimed to explore the relationships between ADHD and BDNF gene polymorphism.
Methods
We conducted a case-control analysis of 202 ADHD subjects and 159 controls, performed a transmission disequilibrium test on 151 trios, and compared the results of a continuous performance test (CPT) according to the genotype of the three single nucleotide polymorphisms (rs11030101, rs6265, rs16917204) in the BDNF gene.
Results
In the case-control analysis, the AA genotype of the BDNF rs11030101 polymorphism was significantly associated with ADHD only in girls (p=0.024, odds ratio=3.00). The T-G-G haplotype was significantly less frequent (p=0.005) and A-G-G was more frequent (p=0.048) in girls with ADHD than in control girls (global p=0.027). A multivariate analysis of variance for commission errors on the CPT showed a significant main effect for the rs11030101 genotype (p=0.026) and an interaction effect of the rs11030101 genotype and gender (p=0.032) in ADHD probands.
Conclusion
These results provide preliminary evidence for a gender-specific association between BDNF and ADHD in the Korean population.
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD), which affects about 5% of school-aged children worldwide,1 is a neurodevelopmental disorder characterized by inattention, hyperactivity, and impulsivity. ADHD causes social, academic, and behavioral problems and persists into adulthood in 30-50% of cases.1 Familial and genetic studies strongly support a role for a genetic component in ADHD pathogenesis, and the estimated heritability is approximately 80%.2
The catecholamine systems have been implicated in the pathophysiology of ADHD, and genes modulating dopamine and norepinephrine system have been studied extensively.2 Nevertheless, evidence from neurobiological and pharmacological research supports the role of neurotrophic factors in ADHD pathogenesis. Given that ADHD is a neurodevelopmental disorder, neurotrophic factors supporting neuronal survival, proliferation, and differentiation, as well as survival and synaptic plasticity in the central nervous system,3 might be involved in ADHD susceptibility. Among neurotrophic factors, brain-derived neurotrophic factor (BDNF), which is critical for the survival and differentiation of midbrain dopaminergic neurons4 and the phenotypic differentiation of locus ceruleus noradrenergic neurons,5 has been a focus.
BDNF messenger RNA is expressed in the mesolimbic dopaminergic system, the prefrontal cortex, the limbic system, and the cerebellum,3 which have been implicated in ADHD pathogenesis. BDNF gene expression is reduced in the frontal cortex of dopamine transporter knockout mice, which is considered as an animal model for ADHD.6 BDNF conditional knockout mice exhibit increased locomotor hyperactivity, which mimics the fundamental behavioral characteristics of ADHD.7 Moreover, BDNF mediates psychostimulant-induced neuroadaptations and locomotor activity through the dopaminergic, serotoninergic, and noradrenergic neurotransmitter systems.8 A recent study reported a positive correlation between plasma BDNF levels and severity of omission errors on the continuous performance test (CPT) in patients with ADHD.9
Previous studies have shown inconsistent results regarding the relationship between the BDNF gene and ADHD. Case-control studies8,10,11 have shown negative results, but in one study,11 a haplotype containing the rs6265 G allele increased significantly in children with ADHD. Family-based association analyses are also not in agreement.10-13 In quantitative trait analysis, there is some evidence of association of parent-rated symptoms and cognitive functions.14 However, no study has investigated the association between the BDNF gene and the results of the CPT.
Thus, we aimed to evaluate the relationships between ADHD and BDNF gene polymorphisms by conducting case-control and family-based association studies of the BDNF gene and by comparing the results of the CPT based on the genotype of the BDNF gene in a Korean sample.
METHODS
The subjects with ADHD and their parents were recruited through the Department of Child and Adolescent Psychiatry at three university-affiliated hospitals in Korea (Seoul National, Kyungpook National, and Chungbuk National Hospitals). All subjects with ADHD met the following inclusion criteria: 1) DSM-IV diagnosis of ADHD; 2) above the 90th percentile in the ADHD Rating Scale-IV (ARS);15 3) T-scores greater than 60 on the attention problems profile of the Child Behavior Checklist (CBCL);16 and 4) intelligence quotient (IQ) higher than 71 on the Korean Educational Developmental Institute's Wechsler Intelligence Scale for Children (KEDI-WISC).17
The control group was recruited from nine elementary schools in Seoul, Incheon, Seongnam, Ulan, and Yeoncheon, Korea. The children selected for the control group 1) did not meet the criteria for the DSM-IV diagnosis of ADHD; 2) scored below the 90th percentile on the ARS; 3) had T-scores of less than 60 on the attention problems profile of the CBCL; and 4) had IQ scores higher than 71 on the KEDI-WISC.
Subjects were excluded from the study if they had one or more of the following: 1) past and/or current history of neurological disorder, including seizure disorder or brain damage; and 2) presence of comorbid psychosis, Tourette's, bipolar, communication, learning, or pervasive developmental disorder.
We obtained written informed consent from the parents of all children who participated in the study and approval from the institutional review boards at each institution where the study was performed.
The diagnoses of ADHD and comorbid disorders of the ADHD subjects were determined using the Korean version of the Schedule for Affective Disorders and Schizophrenia for School-Aged Children-Present and Lifetime Version (K-SADS-PL-K).18 The children in the control group did not meet the criteria for ADHD according to the Korean version of the Diagnostic Interview Schedule for Children Version-IV (DISC-IV).19,20
A computerized CPT was used to measure cognitive functions.21 A visual stimulus was presented every 2 sec for 100 ms, and subjects were asked to respond to a square containing a triangle (target), but not to a square containing a circle or a square (nontarget). The target stimulus was presented in 22.5% of the trials during the first half and in 77.5% of the trials during the second half of the CPT. The four major variables recorded were 1) omission errors, 2) commission errors, 3) response time, and 4) the standard deviations of the response time for correct responses to the target (response time variability).
Genomic DNA was extracted from blood (stored frozen) using the G-DEX™ II Genomic DNA Extraction Kit (Intron, Seongnam, Korea). The detection of a single nucleotide polymorphism (SNP) was based on analysis of primer extension products generated from previously amplified genomic DNA using a chip-based MALDI-TOF mass spectrometry platform (Sequenom, Inc., San Diego, CA, USA). All primers in the PCR and hME reactions were designed using Assay Designer 3.1 (Sequenom) (5'-ACGTTGGATGGCTTGACATCATTGGCTGAC and 5'-ACGTTGGATGTTTTCTTCATTGGGCCGAAC for the rs6265; 5'-ACGTTGGATGACCCACCAGAAAGCTCAATC and 5'-ACGTTGGATGTCCTGTTTCTAATCACAGGG for the rs16917204; 5'-ACGTTGGATGCCTGTAAAACAGGATGGCTC and 5'-ACGTTGGATGATGGCTCCAGGAAAGAG TTC for the rs11030101).
We tested the family-based and case-control associations for each individual polymorphism and haplotype using the standard transmission disequilibrium test (TDT) method in Haploview (http://www.broad.mit.edu/mpg/haploview/) or SNPAlyzer software ver 7.0 (Dynacom, Chiba, Japan). Multivariate analysis of variance (MANOVA) was computed to examine the effect of BDNF genotype and gender on the result of the CPT. SPSS (ver. 15.0) was used for the analysis.
RESULTS
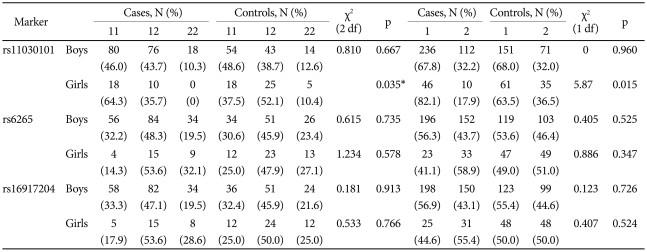
This study included 202 subjects with ADHD (9.0±2.5 years) and 159 normal children (9.0±2.7 years) for the case-control analysis. Table 1 illustrates demographic and clinical characteristics of ADHD and control subjects. The mean ages of the two groups were not significantly different (p=0.924). The gender distribution of the two groups did differ significantly; 174 ADHD subjects and 111 normal controls were boys (χ2=14.67, p<0.001). Thus, the case-control and family-based analyses of boys and girls were conducted separately. The mean age, IQ, inattention, hyperactivity and total scores of ARS, and ADHD subtype were not significantly different between genders (p=0.797, 0.714, 0.531, 0.981, 0.755, 0.088, respectively). Comorbidity with oppositional defiant, conduct, mood, anxiety disorder, and enuresis were not different between genders (p=0.773, 1, 1, 0.087, 0.652, respectively). All participants were ethnically Korean. The family-based analysis included 151 trios consisting of an affected subject and his or her biological father and mother.
The genotype distribution of the three SNPs did not deviate from expectations based on the Hardy-Weinberg equilibrium (p>0.05). In a case-control analysis, the A allele and AA genotype of the rs11030101 were significantly higher in girls with ADHD than in control girls. The odds ratios of the A allele and AA genotype were 2.64 [95% confidence interval (CI)=1.19-5.88] and 3.00 (95% CI=1.14-7.91), respectively. We found no significant differences between the ADHD and control groups in the genotype or allele frequencies for boys or in other polymorphisms (Table 2).
The results of the linkage disequilibrium analysis revealed that the three BDNF SNPs were in strong disequilibrium (rs11030101-rs16917204, r2=0.328; rs16917204-rs6265, r2=0.404; rs11030101-rs6265, r2=0.864). In the haplotype analysis (Table 3), the T-G-G haplotype was significantly less frequent (p=0.005) and A-G-G was more frequent (p=0.048) in girls with ADHD than in control girls (p-value for global test=0.027). We found no significant differences in haplotype frequency in the ADHD and control boys.
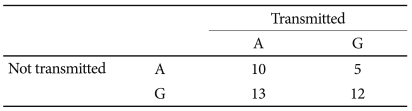
The TDT analysis showed a trend toward the preferential transmission of the A allele of the BDNF rs6265 polymorphism (p=0.059) in girls (Table 4). No preferential transmission was found for boys or in other polymorphisms.
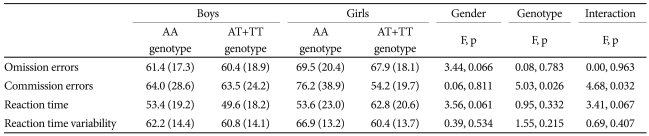
A total of 173 ADHD subjects without missing data were included in the MANOVA to examine the effect of BDNF genotype and gender on the CPT results.
We found no significant differences between excluded and included subjects with regard to demographic and clinical characteristics. The MANOVA for commission errors on the CPT showed a significant main effect of the rs11030101 genotype (F1=5.028, p=0.026) and an interaction effect of the rs11030101 genotype and gender (F1=4.679, p=0.032) in ADHD probands (Table 5).
DISCUSSION
In our case-control and haplotype analyses, BDNF polymorphism was associated with ADHD in girls. The rs11030101 polymorphism was significantly related to commission errors, especially in girls. Thus, the results of this study suggest a gender-specific association of the BDNF gene with ADHD.
This gender-specific association of BDNF is in agreement with the well-known gender differences in the prevalence, course, and clinical features of ADHD.22 Recent studies suggest that many genes including BDNF, or the genetic variations within them, may act differently in males and females.23 Studies that have examined the sexually dimorphic effects of genes in ADHD have also been increasing.24,25
Many of these gender effects may be the result of hormonal influences on gene expression and regulation or other non-genetic factors that are correlated with gender. In the case of BDNF, sex hormones may influence the activity and expression of BDNF-producing cortical neurons. In an animal model, estrogen was reported to regulate BDNF expression by directly interacting with an estrogen response element-like sequence26 and by indirectly modulating GABAergic interneurons.27 Unfortunately, the mechanisms by which estrogen affects the developing human brain are ill defined. However, because estrogen levels differ between genders during the prepubertal period as well as during the prenatal period and after puberty,28 estrogen could contribute to the gender-specific modulation of BDNF gene in children with ADHD.
Moreover, our results are congruent with sexual dimorphism in brain structures involved in the neurobiology of ADHD, particularly the prefrontal cortex. Brain development is known to differ between genders in typically developing children and adolescents, and this difference is more evident in the prefrontal cortex.29 Involvement of the prefrontal cortex is one of the best-replicated findings with respect to brain regions associated with ADHD,30 and there is emerging evidence that the trajectories of early anomalous development of prefrontal cortex in ADHD is gender-specific.22 The BDNF gene has been reported to be associated with volume of prefrontal cortex both in animal31 and in human studies.32
The rs11030101 polymorphism is located within the BDNF intron and has no obvious functional consequences. However, in this study, strong linkage disequilibrium was observed with rs6265, also known as the Val66Met SNP, which causes an amino acid substitution of valine to methionine. Met-BDNF alters intracellular trafficking and activity-dependent secretion in neurosecretory cells and cortical neurons.33
Several limitations of this study should be noted. First, the sample size of this study was relatively small, and only 28 ADHD and 48 control girls were included in the case-control analysis, which was small to avoid a sample bias. Second, there were significant differences between the ADHD and control groups with regard to gender and IQ. Although we performed the case-control and family-based analyses of boys and girls separately, the difference in IQ between the two groups could influence our results. Third, the association of BDNF and ADHD in the case-control analysis and MANOVA did not persist after correction for multiple testing. Fourth, the diagnosis of the ADHD subjects and controls was based on different diagnostic interview tools (K-SADS-PL-K or DISC-IV). However, good diagnostic validities have been established for both instruments, and the same rating scales (K-ARS and CBCL) were applied for diagnosis in both groups. Further studies with a greater number of subjects, especially girls, are required to validate our results.
Despite these caveats, the results of this study provide preliminary evidence for the sexually dimorphic effects of the BDNF gene among Korean patients with ADHD.
Acknowledgments
This study was supported by the Choi Shin Hae New Research Fund of Korean Neuropsychiatry Research Foundation in 2007.