Completion of Chronic Hepatitis C Virus Treatment in Interferon-Induced Major Depressive Disorder with Psychotic Features
Article information
Abstract
Interferon (IFN)-associated psychiatric disorders can be managed without interruption to hepatitis C virus (HCV) treatment. The limited number of cases in the literature reporting psychotic depression as an adverse drug reaction to IFN resulted in discontinuation of HCV therapy. The author reports a case of a 49 year-old man with chronic HCV genotype 1a treated with pegylated interferon-alpha and ribavirin developing major depressive disorder with psychotic features. The patient was successfully treated with both an antidepressant and antipsychotic for this suspected IFN-associated adverse drug effect while continuing 12 months of uninterrupted HCV treatment and subsequently achieving sustained hepatitis C virological response. Although IFN can cause distressing psychiatric disturbances, appropriate treatment with psychotropic agents and careful monitoring allows patients to be maintained on a full course of HCV treatment.
INTRODUCTION
Standard pharmacologic treatment for chronic hepatitis C virus (HCV) infection for a disease that afflicts more than 170 million people worldwide1 is the antiviral combination of pegylated interferon (IFN) and ribavirin (RBV).2 IFN treatment is associated with psychiatric adverse drug reactions3 such as major depressive disorder,4,5 mania,6 psychosis7-9 and suicidal ideation.10 Depending on its severity, discontinuation of IFN may be warranted.7 If monitored closely and managed carefully, however, patients with HCV can be placed on psychotropic medications to treat depressed mood, irritability, and even auditory and visual hallucinations, whereby sustaining HCV treatment.9 In the following report, an HCV-infected patient was treated with a full course of IFN and RBV while being assessed regularly and adequately maintained on an antidepressant and antipsychotic for emergent psychotic depression. The patient eventually achieved a sustained virological response (SVR) with a full course of uninterrupted HCV treatment due to control of psychiatric symptoms.
CASE
A 49 year-old man diagnosed with chronic HCV genotype 1a was screened at the hepatitis C clinic. He was been infected approximately 30 years ago from intravenous drug use which stopped 18 years ago. After starting a 48-week course of HCV therapy he progressively developed major depressive disorder (MDD) with psychotic features requiring pharmacologic intervention.
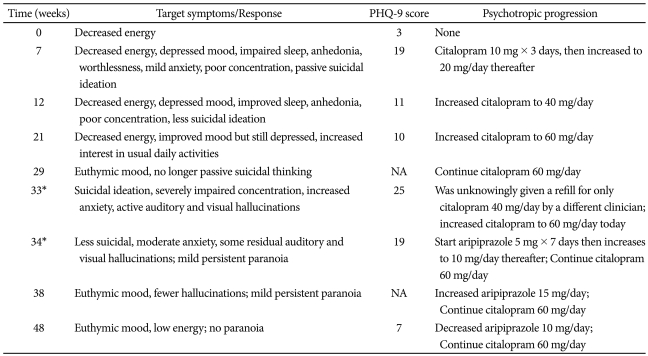
Laboratory findings prior to IFN initiation included an HCV RNA viral load of 2,950,000 copies/mL. Hepatitis B surface antigen and HIV tests were both negative. Hematological findings were unremarkable and baseline TSH was 1.70 uIU/mL (normal= 0.5-3.50). Routine depression monitoring on clinic visit 1 revealed a baseline Personal Health Questionnaire-9 items11 (PHQ-9) score of 3, indicating decreased energy experienced almost daily. Table 1 summarizes depressive symptoms over the course of IFN treatment, which demonstrates a significant increase in the depression score when HCV treatment first began. Pegylated interferon-alpha 180 micrograms once weekly and ribavirin 600 mg twice daily were started. Approximately seven weeks after initiation, the patient began reporting feelings of depressed mood, impaired sleep, poor concentration, and passive suicidal ideation (PHQ-9 score of 19). He insisted, however, that he would never take his own life due to personal religious convictions.
He was counseled about the possibility that his depression could get worse without an antidepressant and subsequently his HCV treatment may have to be discontinued. He therefore agreed to start citalopram. When the dose was eventually increased to 60 mg/day, his mood remained mildly depressed but he no longer reported anhedonia. He requested to be maintained on this maximum dose through week 29.
When the patient returned to clinic for routine follow-up at week 33, it was discovered that he was inadvertently taking a lower daily antidepressant dose of 40 mg for the past two and a half weeks. He started to report severe worsening of mood, passive suicidal ideation, impaired concentration, un-intrusive auditory and visual hallucinations, and mild paranoia around people he was unfamiliar with. Citalopram was immediately increased back to 60 mg/day. The patient denied suicidality and the interdisciplinary team decided that hospitalization was not required. The clinician corroborated the report with the patient's sister and agreed to follow the patient more closely with home telephone calls. The patient agreed to start an antipsychotic and was maintained on aripiprazole 15 mg/day. One week later, suicidal thinking was minimal and hallucinations were less bothersome but still present. Mild paranoid thinking persisted for 7 additional weeks until finally subsiding.
The patient was able to complete the full 48-week course of IFN and RBV indicated for HCV genotype 1b without a decrease in IFN dose, interruption in therapy, or further complications. When added to the HCV medications, both citalopram and aripiprazole were well-tolerated. The PHQ-9 score by the end of treatment was 7, indicating that he had positive treatment response to the antidepressant. Although aripiprazole was discontinued, citalopram 40 mg/day for an additional 6 months with a corresponding PHQ-9 score of 4 was continued. At the 1-year follow-up visit, the patient maintained an SVR and no longer required either psychotropic medication.
DISCUSSION
IFN-induced psychiatric complications are a common reason for HCV treatment discontinuation. There are few reports describing patients who develop symptoms of clinical depression with psychosis occurring after the initiation of IFN and RBV therapy.9,12,13 A case of MDD with psychotic features induced by IFN in a polydrug user describes the patient requiring discontinuation of HCV treatment and the addition of an antidepressant and antipsychotic agent.12 In other instances, patients do not require cessation of HCV treatment when such adverse psychiatric events occur as long as a psychotropic agent is implemented.14 If feasible, concomitant treatment with appropriate psychotropics should be initiated with continuous HCV treatment. Further studies are needed to address the appropriateness of stopping such agents relative to the end of HCV treatment.
Possible neurochemical mechanisms of IFN-induced psychopathology include diminished serotonergic neurotransmi-ssion.15 Hepatic tryptophan dioxygenase degrades tryptophan, the precursor of serotonin. This suggests that as a class of polypeptide cytokines, IFNs possess anti-serotonergic effects and may suppress serotonin synthesis. Further investigation is required to describe depression provocation by IFN.
Depressive symptoms, when inadequately controlled, can lead to secondary psychosis. The psychotic features in this patient were mood-congruent to the depression. Once the depression eventually improved, the psychotic symptoms began remitting. Careful observation was required to ensure that he would not act upon the command auditory hallucinations and paranoia and subsequently harm himself. This required additional close contact with the patient's sister in order to ensure completion of HCV treatment even in the presence of comorbid psychiatric complications caused by the antiviral medications.
Liver function tests monitoring continued and no complications or adverse events were observed with concomitant use of aripiprazole and citalopram. Once-daily aripiprazole was chosen because the patient did not wish to be overly sedated, as he was already feeling fatigue from IFN. A careful balance between minimizing psychotropic polypharmacy and adequately managing substance-induced mood disorder should exist. This is the first reported case of aripiprazole used in the management of IFN-induced psychotic depression where HCV treatment completion resulted in SVR. There is a report on the use of aripiprazole 10 mg/day in a patient who developed IFN-induced psychosis with no noted depression who had to prematurely discontinue HCV treatments.16
Clinicians should work together to manage adverse psychiatric drug reactions in order to sustain HCV-infected individuals on necessary antiviral regimens with the goal of achieving SVR. Early detection and treatment of psychiatric complications induced by IFN can spare HCV treatment. Careful psychiatric symptom assessment of patients using IFN is required in all patients at baseline and as HCV treatment progresses. Immediate identification of serious adverse effects and intervention with appropriate psychotropic treatments allows patients to be managed for psychiatric side effects while being maintained successfully on the difficult treatment regimen for HCV.