Is the Prevalence of the Deficit Syndrome in Schizophrenia Higher than Estimated? Results of a Meta-Analysis
Article information
Abstract
The primary and enduring presence of negative symptoms observed in a relatively homogeneous subgroup of patients with schizophrenia led to the concept of deficit syndrome (DS). Until date, it is considered that 20–25% of schizophrenia cohorts have DS. The aim of this meta-analysis was to determine the current prevalence of DS, including international and most recent studies. Thirteen observational studies met the inclusion criteria, comprising 2092 patients from eight countries. Pooled proportion of the DS subgroup was 32.64%, higher than previously reported. Based on our outcomes, up to one-third of patients with schizophrenia might have idiopathic and stable negative symptoms. This significant proportion of patients should be well represented in clinical trial's samples.
INTRODUCTION
Negative symptoms are a heterogeneous clinical construct which constitute a core psychopathological domain of schizophrenia.1 They can be stable or transient and have been divided into primary, when they are intrinsic to the illness, or secondary, if they originated from confounding conditions (medication side effects, psychotic symptoms, depression, substance abuse, or environmental deprivation).12 Nonetheless, they are associated with marked functional disability, diminution of quality of life and increased burden on patients' carers.3456
The primary and enduring presence of negative symptoms observed in a relatively homogeneous subgroup of patients with schizophrenia led to the concept of the deficit syndrome (DS).2 Studies comparing differences between a DS group and a non-deficit group have contributed to the hypothesis that deficit schizophrenia could be a separate disease.78910 However, despite evidence suggesting that DS represents a valid taxon, it has not been considered either as a subtype or as an independent psychotic disorder in the fifth Diagnostic and Statistical Manual of Mental Disorders.11
Distinguishing primary from secondary negative symptoms in DS requires good clinical care and bears important therapeutic implications.12 Although there are several specific tools for the assessment of negative symptoms, the Schedule for the Deficit Syndrome (SDS)13 is considered the gold-standard instrument to evaluate those symptoms as primary and stable.12 This scale defines DS on the basis of clinical criteria extracted from patients' past and present mental states. It consists of a semi-structured interview that also collects information from relatives and allows an operative categorisation between deficit and non-deficit groups.14 According to this methodology, about 20–25% of schizophrenia cohorts have DS.115
When the SDS is not available (usually it is not feasible in large epidemiological samples), the Proxy for the Deficit Syndrome (PDS) has been proposed as an alternative method for assessment.16 PDS classifies the subjects into deficit or non-deficit groups based on a specific profile on the Brief Psychiatric Rating Scale (BPRS) or the Positive and Negative Syndrome Scale (PANSS).17 However, DS proportions vary from 2% to 34% in different studies when the PDS scale is used,18 which indicates that it might not be a reliable instrument for an accurate categorisation.141920
To our knowledge, the epidemiological rate of deficit schizophrenia has not been calculated using a meta-analytic framework and the reference data available in the literature correspond to estimates conducted by SDS authors in the United States (mostly during the 1990s).101621 Therefore, the aim of this meta-analysis was to determine the current prevalence of DS (assessed through SDS), including international and most recent studies.
METHODS
Search strategy
The methodology was based on the guidelines outlined by the Meta-analysis of Observational Studies in Epidemiology (MOOSE) recommendations.22 To identify the relevant literature, a combined search in PubMed, Medline, EMBASE and Google Scholar was conducted. Considering that the evaluated period could condition the outcomes and with the aim of obtaining a prevalence rate as close to the present as possible, we decided to limit the search for articles to a recent period in time between 2010 and 2015. We also carried out a hand-search for citations from the retrieved studies. The following keywords were used: ‘schedule for the deficit syndrome’ combined with ‘deficit schizophrenia’, ‘deficit syndrome’, ‘negative symptoms’.
Inclusion and exclusion criteria
The first author (ÁL-D) screened the titles and abstracts, then all authors independently reviewed the full texts of the selected articles. Studies were included if they met all of the following criteria: 1) written or translated in English; 2) published in a peer-reviewed journal; 3) related to any schizophrenia topic with a deficit/non-deficit categorisation; 4) authors did not use purposive sampling unless they reported in the article the proportion of patients with DS among the total sample; and 5) SDS was the instrument employed to enabling the diagnosis of DS given that is the gold-standard measure for separating primary and secondary negative symptoms in deficit schizophrenia. In cases where the samples overlapped, the article with the largest sample size was included. Reviews, editorials, opinion articles and research studies with fewer than 10 subjects were excluded.
Outcome measure, data extraction and quality assessment
In this epidemiological analysis, the standardised outcome measure was the proportion of patients with deficit schizophrenia, therefore only data concerning the prevalence of DS among the sample of each study were extracted. Other clinical and demographic variables (such as gender, season of birth, age of onset, duration of illness and symptoms severity) were not considered. Although there is no clear consensus on the method to be used to appraise quality assessment in observational studies,23 we used a simple objective rating system based on the meta-analysis conducted by Paulson et al.24 Studies were thus coded on a scale of 0 to 10, with 2 points each assigned for recruitment strategies (probability vs nonprobability sampling), inclusion and exclusion criteria (clearly stated or not), ethnic diversity (≥20% minority), educational diversity (≤80% at 1 educational level) and the response rate (reported at ≥60%).24 Two independent reviewers (ÁL-D and IL) performed the quality assessment, with a third reviewer (GL) consulted in case of any disagreement.
Data analysis
A meta-analysis on the selected papers was conducted to obtain an overall weighted prevalence of DS. Data from each individual study were pooled using a DerSimonian-Laird proportion meta-analysis.25 As it was not clear whether the outcome measure was affected by the factors used in the quality assessment, weighting of the included articles according to that rating system was not performed. However, a second analysis was conducted excluding those studies with the lowest quality scores (<6 points) to determine whether potential methodological weaknesses could have influenced the meta-analytic estimates. Statistical procedures were performed with MedCalc Statistical Software version 15.8 (MedCalc Software, Mariakerke, Belgium). Data extraction and analysis were carried out by the main author (ÁL-D) and checked by the others.
RESULTS
Search results
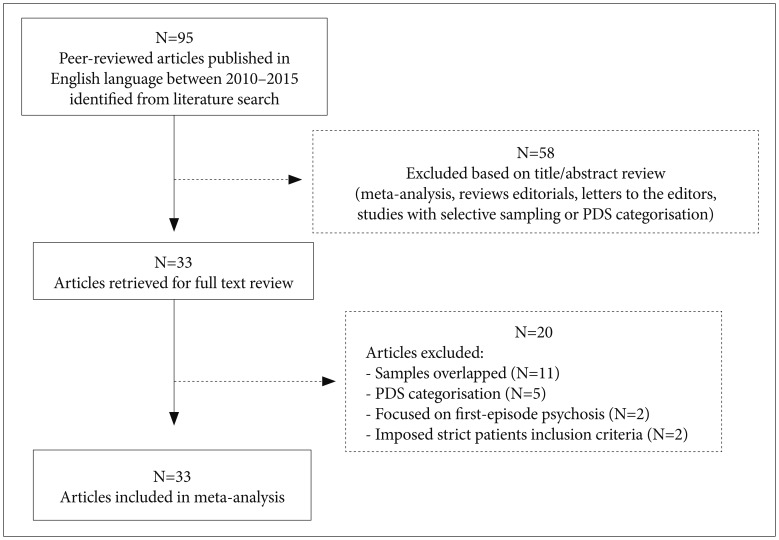
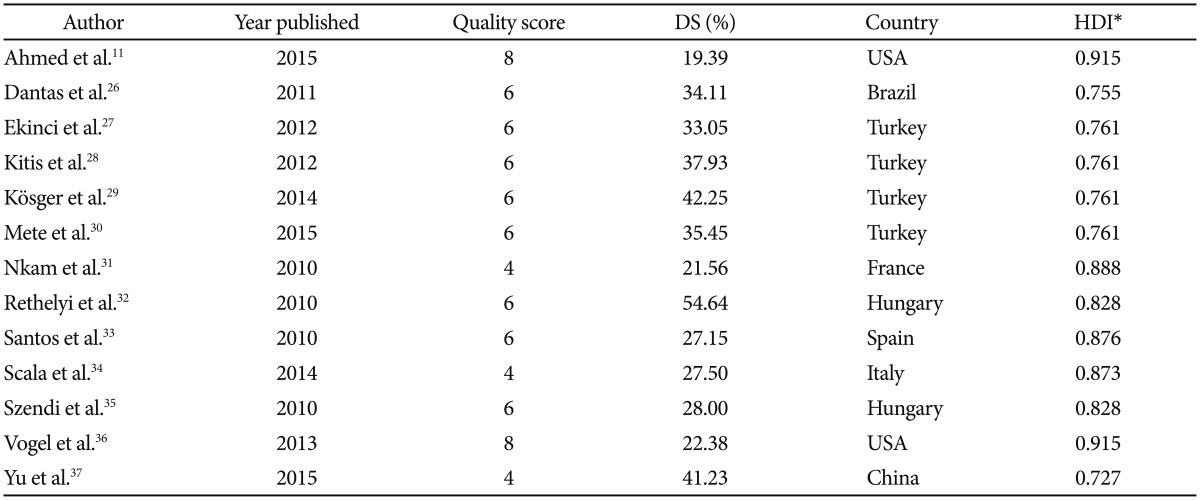
The initial literature search yielded a total of 95 peer-reviewed articles published in English language journals. A subset of 33 articles was selected for consideration after screening the titles and abstracts. These potentially relevant studies were full-text reviewed to determine eligibility and to extract prevalence data of DS. Finally, 13 articles met the inclusion criteria (Figure 1).15262728293031323334353637 The studies comprised 2,092 patients from eight countries (Brazil, China, France, Hungary, Italy, Spain, Turkey, and the United States), with subjects aged between 18 and 69 years old. The quality assessment of the articles yielded scores rated between 4 and 8 points (Table 1).
As heterogeneity was invariably high, random-effects meta-analyses were performed on the estimates to generate summary values. The pooled proportion of the DS subgroup was 32.64% (95% CI=25.39 to 40.34) (Figure 2). Removing the studies with the lowest quality ratings (<6 points), the meta-analytical estimate of DS varied by only 0.58% (from 32.64% to 33.22%). Therefore, the results remained essentially unchanged in direction and magnitude.
Excluded studies
A total of 82 studies did not meet the inclusion criteria (Figure 1). Sixty-seven articles covering reviews, meta-analysis, editorials, letters to the editors and studies with selective sampling or PDS categorisation were excluded. Two papers were refused because they imposed strict patients inclusion criteria and two others were rejected given that the research was focused on first-episode psychosis. Eleven articles were omitted because it was found that samples may not have been entirely independent of each other. Of those, five corresponded to studies carried out at the Maryland Psychiatric Research Center.
DISCUSSION
The proportion of deficit schizophrenia found in this meta-analysis (32.64%) was higher than that originally reported by the authors of the SDS scale (20–25%). This may be because we included articles from several countries in which the profiles of the attended population and the types of received treatment could have been different. This fact can be interpreted at the same time as a methodological limitation and as one of the strengths of this research since it provides a more comprehensive overview of the worldwide prevalence of DS. Thus, we have been able to observe that studies carried out in countries with lower Human Development Index38 were those that yielded higher DS frequencies (Table 1). Furthermore, by reviewing other meta-analyses related to DS that included earlier articles than those examined by us, we found that deficit schizophrenia rates were in many cases also higher than expected.192039
Based on our outcomes, up to one-third of patients with schizophrenia might have idiopathic and stable negative symptoms for which no effective treatment exists yet.4041 Hence, this high prevalence of DS should steer clinicians towards focusing more on the task of distinguishing between primary and secondary negative symptoms, as well as trying to minimise secondary causes. This difficult task might often be neglected and could lead to false optimism in the interpretation of some clinical trials, where observed improvement in terms of negative symptomatology42 would be down to nothing more than reductions in secondary sources.15
Moreover, it is also important to stress that the term DS should be applied only to patients meeting the SDS diagnostic criteria,9 and its assessment precises a methodology that is not usually feasible for randomised controlled trials in schizophrenia.43 In those, measures of negative symptoms rely upon the PANSS negative factor or the BPRS negative symptom subscale or the Scale for the Assessment of Negative Symptoms (SANS). Such scales could be useful for detecting subjects with persistent negative symptoms44 but are not appropriate for categorising deficit schizophrenia. For this reason, patients with DS have not been well identified in those studies and therefore outcomes may not be generalisable for that subgroup.
In a personalised medicine paradigm, subjects with deficit schizophrenia are likely to benefit from specific interventions, and this can only be achieved by conducting clinical trials with representative samples of this subset of patients. Our contribution is to indicate that the prevalence of DS, according to the most recent international studies, may be higher than the estimated one. This could encourage many investigators to carry out further studies which help to deepen the understanding of primary negative symptoms as well as the development of specific treatments for them, undoubtedly one of the greatest challenges in schizophrenia research.