Modulation of Electrophysiology by Transcranial Direct Current Stimulation in Psychiatric Disorders: A Systematic Review
Article information
Abstract
Objective
Transcranial direct current stimulation (tDCS) is a non-invasive neuromodulation technique increasingly used to relieve symptoms of psychiatric disorders. Electrophysiologic markers, such as electroencephalography (EEG) and event-related potentials (ERP), have high temporal resolution sensitive to detect plastic changes of the brain associated with symptomatic improvement following tDCS application.
Methods
We performed systematic review to identify electrophysiological markers that reflect tDCS effects on plastic brain changes in psychiatric disorders. A total of 638 studies were identified by searching PubMed, Embase, psychINFPO. Of these, 21 full-text articles were assessed eligible and included in the review.
Results
Although the reviewed studies were heterogeneous in their choices of tDCS protocols, targeted electrophysiological markers, and disease entities, their results strongly support EEG/ERPs to sensitively reflect plastic brain changes and the associated symptomatic improvement following tDCS.
Conclusion
EEG/ERPs may serve a potent tool in revealing the mechanisms underlying psychiatric symptoms, as well as in localizing the brain area targeted for stimulation. Future studies in each disease entities employing consistent tDCS protocols and electrophysiological markers would be necessary in order to substantiate and further elaborate the findings of studies included in the present systematic review.
INTRODUCTION
Transcranial direct current stimulation (tDCS) is a noninvasive neuromodulation technique that delivers low amplitude direct current across the scalp [1]. Though their underlying mechanism has proven more complicated, tDCS anode and cathode can simultaneously activate and suppress activity of targeted brain areas [2,3]. For this reason, increasing attention has been paid to the potential utility of tDCS in treating psychiatric disorders that exhibit unbalanced hypo- or hyperactivity of brain circuits, such as major depressive disorder, schizophrenia, and obsessive-compulsive disorder [2,4,5]. Moreover, the relatively high safety and tolerability, cost-effectiveness, and ease of administration associated with tDCS have further supported its clinical utility in psychiatric disorders [6-8].
In addition to the clinical utility of tDCS for treatment of psychiatric disorders, investigating tDCS effects on psychiatric symptoms in association with plastic changes of the related brain circuitry could offer valuable information for better understanding the pathophysiological mechanisms of dysfunctional neural circuits in psychiatric disorders. Previous studies found tDCS to induce plastic changes of neuronal activity by modulating resting membrane potential and N-methyl-D-aspartic acid receptor (NMDAR) activity [9]. Furthermore, it has been also reported that anodal tDCS produces excitation-like long term potentiation (LTP), whereas cathodal tDCS induces inhibition-like long term depression (LTD) [10,11].
In studying tDCS-induced plasticity in psychiatric populations, utilizing electrophysiological measures of electroencephalography (EEG) and event-related potentials (ERP) demonstrate strengths. As measures directly reflecting neural activity during both resting and information processing states with high temporal resolution at the millisecond level, EEG and ERPs have long been employed in examining the altered neural processing associated with psychiatric disorders [12]. These measures are also powerful tools for measuring plastic changes of brain after specific intervention, such as following medication, cognitive exercise, and tDCS [13-16]. Therefore, investigation of tDCS effect on EEG and ERPs may provide further information on pathophysiologic mechanism underlying psychiatric disorders, as well as a serve as a guide for personalized neuromodulation in response prediction and selection of target areas. We performed the present systematic review to identify electrophysiological markers reflecting tDCS effects on plastic brain changes in psychiatric disorders, that, in turn, may serve as a biomarker for pathophysiology of psychiatric disorders.
METHODS
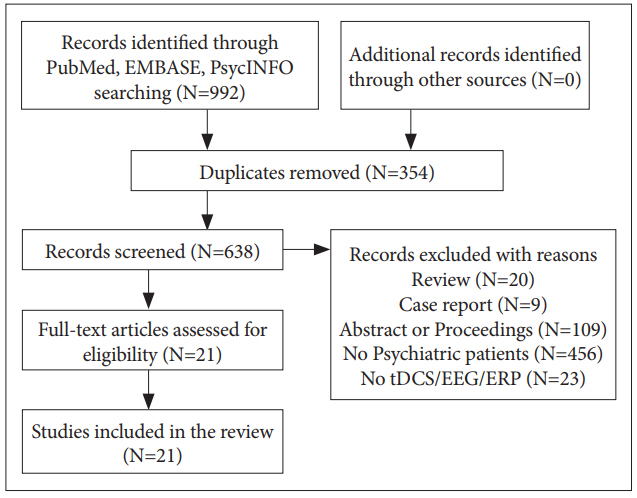
A systematic search strategy was used to identify relevant studies. Two independent researchers (M.K. and Y.B.K.) each conducted literature searches in PubMed, Embase, and PsycINFO databases for articles published during the period spanning from database inception to February 2017. The following Medical Subject Heading (MeSH) categories were used for the search: (electroencephalography OR EEG OR event related potential OR event-related potential OR ERP) AND (transcranial direct current stimulation OR tDCS). A total of 638 articles were found, and of these, only the studies satisfying the following criteria were included in the current review: 1) articles written in English; 2) original articles or short communications in peer-reviewed journals; 3) studies that included psychiatric patients; and 4) studies with outcome measured with EEG or ERP. The process for literature search and screening are presented in Figure 1.
RESULTS
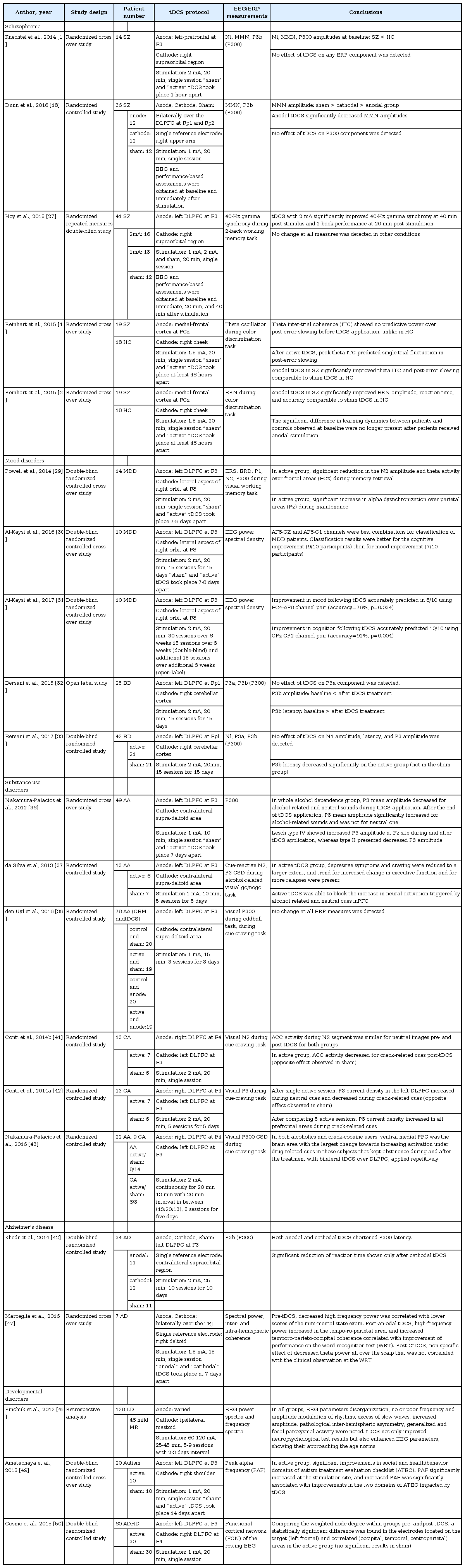
Number of selected studies was 5 for schizophrenia, 5 for affective disorders, 6 for substance use disorders, 2 for Alzheimer’s disease (AD), and 3 for developmental disorders. Summary of studies pooled for review are shown in Table 1.
Schizophrenia
The 5 studies examining patients with schizophrenia applied anodal tDCS to modulate frontal cortical functions, although tDCS protocols used in each study showed slight variations. Of these, two studies examined tDCS effects on early and late auditory processing and reported inconsistent results [17,18]. Alterations in the N1 and mismatch negativity (MMN), which reflect pre-attentive auditory processing, and in P3b or P300, which represents working memory update of change and attention, have all been reported in patients with schizophrenia [19-21]. Knetchel et al. [17] applied anodal tDCS over the left prefrontal cortex in 14 schizophrenia patients and examined effects on N1, MMN, and P3b ERP components. In this randomized cross over study, a single application of tDCS was found to have no acute effects on the ERP measures and associated auditory information processing in patients with schizophrenia. In a more recent randomized controlled study, Dunn et al. [18] applied bilateral prefrontal tDCS on regions associated with auditory processing system in 36 patients with schizophrenia (12 patients in anodal, cathodal, sham group each) and examined effects on MMN and P300 ERP components. Compared to baseline, i.e., before stimulation, MMN amplitudes were found decreased post-stimulus for anodal tDCS. For P300 amplitudes, neither the main effects of time and condition nor their interaction were found significant. Results from Dunn et al. [18] study suggest that bilateral prefrontal tDCS can engage neural systems underlying the impaired auditory processing in schizophrenia patients via the modulation of MMN, whose association with the pathophysiological mechanism, clinical symptoms, as well as general functional status of schizophrenia are well known [22-24]. Thus, although the two aforementioned studies did not measure or provide changes in psychotic symptoms following tDCS application, the reported MMN modulations following a single session of tDCS suggest tDCS as an potentially effective treatment option for improving symptoms and functioning in patients with schizophrenia.
The remaining three studies examined the effects of tDCS on higher cognitive functions, such as working memory, adaptive control, and learning ability, in relationship with modulation of EEG/ERPs that reflect such cognitive functions. Although cognitive dysfunction has been considered a major symptom domain of schizophrenia, the direct measurement of cognitive functioning is confounded by factors such as motor speed, psychotic symptoms, and fatigability. EEG and ERPs can effectively control these confounders and provide more objective evaluation of cognitive functioning with high temporal resolution [25,26]. Hoy et al. [27] examined whether improvements in working memory following tDCS on the left dorsolateral prefrontal cortex (DLPFC) would be reflected as changes in 40-Hz gamma synchrony during 2-back working memory task. Authors reported significantly improved working memory performance, as well as a significant decrease in gamma event-related synchronization. Results from this study provided preliminary evidence for enhanced working memory following tDCS, which restored normal gamma oscillatory function in schizophrenia. Reinhart et al. [28] demonstrated aberrant oscillatory activity in the frontal cortex to underlie deficits in adaptive control in schizophrenia patients. The effect of anodal tDCS over the medial frontal cortex on improving neurophysiological signature of adaptive control provided causal evidence for executive control. In patients with schizophrenia, tDCS was found to modulate the temporal structure of low-frequency oscillations and synchrony and to improve behavioral response of post-error slowing to a level of healthy comparison group during color discrimination task performance. Using the same tDCS protocol, Reinhart et al. [16] also examined whether deficit learning in schizophrenia patients could be improved via increasing error-related negativity (ERN) and demonstrated improved learning rate, as well as the neural signature of prediction error signaling. Results from these randomized cross over studies all together suggested tDCS as a possible treatment option for cognitive enhancement in patients with schizophrenia.
Affective disorders
For studies in affective disorders, 3 studies pertained to major depressive disorder (MDD) and 2 to euthymic bipolar disorder [29-33]. In all the 5 studies, anodal tDCS was applied over the left DLPFC, and in the 2 studies in euthymic bipolar patients, cathodal tDCS was additionally applied on the right cerebellar cortex. To examine the effects of applying anodal tDCS over the left DLPFC in MDD patients, Powell et al. [29] investigated the N2 ERP component and event-related spectral changes during a visual working memory task performance. In this double blind sham controlled crossover study, intervention dependent effects were found, in that active tDCS session resulted in significantly reduced N2 amplitude and theta activity over frontal areas during memory retrieval, as well as in significantly increased alpha over occipital-parietal areas during maintenance. These observed effects on EEG components, which are considered to have a source in medial frontal cortex, suggest tDCS to affect cortical functioning beyond focal changes at the stimulation site. Two studies by Al-Kaysi et al. utilized machine learning methods in EEG data of 10 MDD patients to predict clinical outcomes following tDCS applications over the left DLPFC [30,31]. The authors demonstrated that a multichannel deep belief network can be used to accurately classify between the EEG data that was recorded after active and sham tDCS [30] and identified features from the baseline resting-state EEG that differentiate patients who respond to tDCS treatment from those who do not [31]. Al-Kaysi et al. [30,31] also found frontal channels to perform better in predicting clinical scores, while the parietal-occipital channels better in cognition score. These studies demonstrated the feasibility of EEG-based classification that might help better select patients who would further benefit from tDCS treatment in both alleviating depressive symptoms and neurocognitive functioning. Bersani et al. examined the effects of repeated prefrontal-excitatory and cerebellar-inhibitory tDCS on P300 novelty task in euthymic bipolar patients [32,33]. P3b reflects working memory update of change while subjects pay attention to stimulus, while P3a is elicited by unexpected stimulus and is thought to reflect automatic reorienting [34]. P3 complexes were found associated with higher-order cognitive processing, and their latency suggest the need of cognitive efforts while performing the task [34,35]. These two studies by Bersani et al. that psychophysiologically evaluated neurocognitive functioning differed in terms of their sample size (25 vs. 42), designs (open-label vs. double blind placebo-controlled), and electrode positions used (F3 vs. FP1). Nonetheless, both studies consistently reported decreased P3b latency, and their results suggested the potential utility of prefronto-cerebellar tDCS as an inexpensive, convenient, and painless add-on therapy for treating cognitive dysfunctions in euthymic bipolar disorder patients.
Substance use disorders
Three studies examined ERP components in patients with alcohol dependence for effects of anodal tDCS applied over the left DLPFC [36-38]. Nakamura-Palacios et al. [36] examined effects of tDCS in 49 alcoholic subjects, who were further subgrouped according to Lesch’s typology. Lesch’s typology distinguishes 4 subtypes of alcohol-dependent patients based on the patient’s family history, personal psychopathology, and theoretical neurobiological background [39]. In this randomized cross over study, cognitive and electrophysiological measures demonstrated tDCS-induced frontal activity enhancement specifically in Lesch IV alcoholics, who are associated with pre-morbid cerebral defects, behavioral disorders, and a high social burden [40]. For this sub-group, in particular, increased P3 amplitude during cue-induced auditory paradigm and improved frontal assessment battery scores were reported. Building upon this study, da Silva et al. [37] assessed the effects of repeated tDCS in 13 alcoholic subjects enrolled in a randomized clinical trial. The study reported tDCS effects on craving, ERPs (cue reactivity), and cognitive functions in line with the previous literature, and extended the finding to mood improvement, as well. However, with a trend for more relapses also reported in this study, results all together indicated the need to carefully assess the clinical value of tDCS in future studies. den Uyl et al. [38] investigated effects of tDCS and cognitive bias modification (CBM) in 78 hazardous drinkers. The authors measured not only behavioral indices, such as craving, implicit associations, and approach tendencies, but also visual P300 ERP components elicited by oddball and cue reactivity paradigms. This randomized controlled study demonstrated a specific decrease in cue-induced craving (i.e., not in overall craving), and reported neither behavioral nor electrophysiological effects of repeated CBM and/or tDCS. The remaining three studies examined in in crack-cocaine uses the effects of applying bilateral tDCS with cathodal stimulation over the left DLPFC and anodal stimulation over the right DLPFC [41-43]. In particular, Conti and Nakamura-Palacios investigated tDCS effects on the cue related N2 ERP component of 13 crack-cocaine addicted subjects [41]. In this study, applying a single session of prefrontal tDCS was found to specifically modulated anterior cingulate cortex (ACC) activity of N2 during exposure to drug cues in users and indicated tDCS as a promising adjunctive treatment for addiction. The same authors reported the effects for both single and repetitive applications of bilateral tDCS on P3 ERP component during cue reactivity task in 13 crack-cocaine users [42]. This study reported both single and repeated tDCS application to impact cognitive processing of crack-related cues in prefrontal areas as reflected by change in P3 current source density (CSD). Compared to the effect of single dose, however, that of repetitive tDCS extended beyond the DLPFC to the fronto-parietal cortex, orbitofrontal cortex, and ACC. Nakamura-Palacios et al. [43] reported electrophysiologic effects of repetitive bilateral tDCS in 22 alcoholics examined in Klauss et al. [44] study and 9 crackcocaine users in Conti et al. [42] study. In both alcoholics and crack-cocaine users, the ventral medial prefrontal cortex (VMPFC) was found as the brain area with the largest cue-related P3 CSD change towards increasing activation under drug-related cues in subjects who kept abstinence during and after tDCS treatment. The authors suggested the bilateral DLPFC tDCS to reduce relapses and craving to the drug use and to increase VMPFC activation under drug cues, which may be of a great importance in the control of drug use in drug addiction.
Alzheimer’s disease
Khedr et al. [45] examined long-term efficacy of repeated cathodal, anodal, or sham tDCS over the left DLPFC in the neurorehabilitation of 34 AD patients. Auditory P300 was used as a biological marker of AD because its latency is known to be pathologically increased in AD, meaning that AD patients have less cognitive reservoir and need more effort in performing working memory update of change [46]. The double-blind randomized clinical trial demonstrated repeated sessions of tDCS, both cathodal and anodal, to not only improve cognitive function, but also reduce the P300 latency. The study’s results suggest that tDCS over the left DLPFC may enhance cognitive functioning in AD patients by modulating P300 mechanism associated with cognitive reservoir and need of effort. In another study, Marceglia et al. [47] investigated effects of bilateral temporo-parietal tDCS on quantitative EEG (QEEG) in 7 AD patients. This study reported anodal tDCS to induce significant increase of high-frequency power in the temporo-parietal area, as well as of temporo-parieto-occipital coherence correlated with improvement in the performance of word recognition test. This finding of partial reversal in the abnormal pattern of EEG activity observed in AD patients following tDCS application suggests tDCS benefits in AD during working memory tasks supported by the modulation of cortical activity.
Developmental disorders
Three studies examined tDCS induced modulation of resting EEG in children with developmental disorders. Pinchuck et al. [48] conducted retrospective analysis of tDCS effects on neuropsychological measures and QEEG parameters in children with learning disorders and mild intellectual disability. Although tDCS and EEG protocols across subjects varied, due to the study’s retrospective nature, the authors showed tDCS as not only improving neuropsychological test results but also enhancing EEG parameters to approach the age norms. Amatachaya et al. [49] examined the effects of anodal tDCS over the left DLPFC on peak alpha frequency (PAF), which is known to be significantly decreased in autism, and on clinical symptoms evaluated by autism treatment evaluation checklist. This randomized crossover controlled study demonstrated improvements in social and health/behavior domains of ATEC associated with significant increase in PAF following active tDCS. To explore the potential utility of tDCS in treatment of ADHD, Cosmo et al. [50] assessed the effects of anodal tDCS over the left DLPFC on brain connectivity using the functional cortical network (FCN) based on EEG activity. The study reported increased functional brain connectivity following tDCS, as well as spreading of its modulatory activity.
DISCUSSION
We reviewed 21 articles reporting changes in EEG or ERP measures following tDCS application in psychiatric patients. Our primary aim was to identify electrophysiological markers reflecting the tDCS induced plastic brain changes in association with symptomatic improvement. Such markers can provide valuable information regarding not only the pathophysiology of psychiatric disorders, but also the individualized neuromodulation strategy in psychiatric patients. Subtle change detected by EEG or ERPs may come precede and forecast the later symptomatic improvement, which may not be sufficiently enlarged for detection following a single or small number of tDCS sessions. Furthermore, EEG or ERP has strengths in evaluating the changes in cognitive functioning following tDCS application, because these measures provide more objective biological evaluation of cognitive functioning with high temporal resolution.
In this systematic review, we found that tDCS with EEG or ERPs have been utilized in patient populations of various psychiatric disorders, including schizophrenia, major depressive disorder, euthymic bipolar disorder, alcohol and/or crack-cocaine addiction, AD, learning disorders, mild intellectual disability, autism, attention deficit and hyperactivity disorder. In schizophrenia, ERP components such as MMN, P3b, ERN have been suggested as biomarkers of pathophysiology because they reflect altered neurotransmission, functional connectivity, cognitive dysfunction, as well as clinical symptoms, associated with the disorder [22,23]. Thus, these ERP components have been effectively employed in predicting the course of outcome in schizophrenia and even in prodromal psychosis [19,51,52]. In line with previous studies, result of this systematic review suggests EEG/ERPs modulated by tDCS to reflect the underlying plastic neural changes that accompany or forecast the forthcoming symptomatic improvement by tDCS in patients with schizophrenia. The findings in patients with MDD that the topological pattern of EEG spectra effectively predict responders of tDCS may aid the selection of clinical population for tDCS application [30,31]. In bipolar disorder and AD, impaired P3 complexes reflected specific cognitive dysfunction, such as working memory deficit or pre-attentive processing, evident in the affected patient population. However, the impairments were normalized with cognitive enhancement after tDCS. These findings suggest that plastic neural change by tDCS were reflected in the modulation of EEG/ERPs, that, in turn, consisted improvement of higher-order cognitive functioning. In substance use disorders, altered ERPs were related with substance cue induced craving that normalized with tDCS application, showing that tDCS in addictive disorders may augment substance addiction treatment by modulating neural circuit of craving [37,41-43].
Although with varying protocols, tDCS studies in these psychiatric populations employed a region of interest in the prefrontal cortex, especially the left DLPFC. The effect of tDCS on EEG and ERPs were investigated mainly in relations to cognitive functioning in schizophrenia, mood disorders, AD, and developmental disorders, while the focus primarily lied in the cue-induced craving in substance use disorders. Of the 21 studies reviewed, ten studies examined EEG or ERPs changes after a single session of tDCS, 5 studies after no more than 5 sessions, and 6 studies after more than 10 sessions. Irrespective of targeted EEG/ERP parameters or disease entities, 19 studies reported positive results (i.e., changes in EEG/ERPs following tDCS application and/or their association with symptomatic/cognitive improvement), suggesting that even a single tDCS session can effectively modulate brain plasticity, which is reflected by EEG or ERP in psychiatric disorders and may be relevant to the mechanisms underlying symptomatic or cognitive improvement. However, owing to possible publication bias, cautious interpretation is warranted in reading this systematic review.
In strong support of findings in the current systematic review, our recent study in patients with schizophrenia demonstrated tDCS-induced P50 change and associated improvements in auditory hallucination [53]. Twice a day, for 5 consecutive weekdays, schizophrenia patients with treatment refractory auditory hallucination received tDCS with anodal electrode on the left DLPFC and cathodal electrode on the left temporoparietal junction (TPJ). Auditory hallucination symptom severity and P50 sensory gating were assessed before and after the tDCS application. Improvement in auditory hallucination following tDCS was found significantly correlated with tDCS-induced P50 change. Our results indicated P50 sensory gating as a promising marker that may help reveal the mechanisms underlying tDCS effect on auditory hallucination in schizophrenia.
In this systematic review, number of included studies which tested tDCS effect on EEG/ERPs was too small to draw comprehensive conclusions. Each study investigated different EEG/ERPs in different subject population with different tDCS protocols. However, despite such heterogeneity, the included studies strongly support EEG/ERPs to sensitively reflect tDCSinduced brain changes. Taken together, tDCS and electrophysiology may serve useful and sensitive tools for investigating pathophysiology of psychiatric disorders, as well as for selecting regions targeted for neuromodulation in psychiatric disorders. Future studies with consistent tDCS protocols and electrophysiological markers in each disease entities would be strongly suggested to convince and elaborate the findings of studies included in this systematic review.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (Grant no. NRF-2015R1C1A1A01053988).