Plasma Levels of IL-23 and IL-17 before and after Antidepressant Treatment in Patients with Major Depressive Disorder
Article information
Abstract
Objective
Cytokines are believed to have a role in the pathophysiology of major depression. The alteration in levels of pro-inflammatory cytokines [interleukin 1β (IL-1β), IL-2, IL-6, IL-12, interferon γ, and tumor necrosis factor α] in major depression supports the cytokine hypothesis of this illness. IL-23 and IL-17 are also pro-inflammatory cytokines, but few studies have focused on their role in major depression. This study investigated the potential role of the IL-23 and IL-17 axis in major depression.
Methods
Plasma IL-23 and IL-17 levels were measured in 26 major depressive disorder (MDD) patients before and after 6-week treatment with antidepressants; these levels were measured in 28 age- and sex-matched normal controls. Depression severity was assessed using the Hamilton Depression Rating Scale (HDRS). IL-23 and IL-17 plasma levels were estimated using quantitative enzyme-linked immunosorbent assay.
Results
Pre-treatment plasma levels of IL-23 and IL-17 in MDD patients were not significantly different from those of normal controls. In MDD patients, IL-23 and IL-17 levels after 6 weeks of antidepressant treatment were not different from the baseline levels. There was no significant correlation between changes in the cytokine levels and changes in the HDRS scores representing the severity of depression.
Conclusion
The present study does not support a potential involvement of IL-23 and IL-17 axis in major depression. Replication and extension using a larger sample are required.
INTRODUCTION
Accumulating evidence suggests that dysregulation of immune system, particularly the cytokine system, is associated with the pathophysiology of major depressive disorder (MDD).1-3 Although the central nervous system affects the immune system via the autonomic nervous system and neuroendocrine system, the immune system inversely affects the central nervous system, via a cytokine network secreted by immune cells, to control behaviors and emotions.4 Cytokines are both immunoregulators and modulators of neural functions. Pro-inflammatory cytokines [including interleukin 1 (IL-1), IL-6, tumor necrosis factor α (TNF-α), and interferon γ (IFN-γ)] play important roles in the central nervous system, such as controlling neuronal and glial activation, proliferation, differentiation, as well as affecting neuronal plasticity, synaptogenesis, and tissue repair.4
Several studies suggest that pro-inflammatory cytokine overexpression in the brain is an important factor in MDD.5 Some studies indicate that levels of monocytic pro-inflammatory cytokines (IL-6 and TNF-α) are significantly higher in MDD patients and associated with immunological dysregulation in MDD.6,7 Moreover, the T-helper 1 (Th1; IL-2 and IFN-γ) and Th2 (IL-4) cytokine imbalance seems to play crucial roles in MDD pathogenesis.8
IL-17 is one of the Th17 cell-secreted cytokines, which include IL-17A, IL-17F, IL-6, IL-22, and granulocyte macrophage colony-stimulating factor (GM-CSF), whereas IL-23, along with transforming growth factor β (TGF-β), is involved in the differentiation of naïve CD4+ T cells into Th17 cells in a pro-inflammatory context.9 In the presence of IL-23 and TGF-β, naïve CD4+ T-cells become IL-17-producing Th17 cells via the transcription factor retinoic acid receptor-related orphan receptor γt. IL-17 activates fibroblasts to produce proinflammatory cytokines such as IL-6 and TNF-α, resulting in inflammation and tissue destruction in various autoimmune disorders.10 Thus, both IL-23 and IL-17 form an axis through Th17 cells, playing a key role in immune activation and immunopathogenesis of a number of diseases.11 There have been many studies suggesting that Th17 cells have a crucial role in several inflammatory responses and have a close association with autoimmune diseases. The IL-23/IL-17 axis is associated with several inflammatory conditions such as inflammatory bowel disease (IBD),12 psoriatic arthritis,13 multiple sclerosis,14 autoimmune myocarditis,15 and rheumatoid arthritis.16 There is some evidence that IL-17 is the major regulator of central nervous system autoimmunity.17
There have been reports that patients with psoriatic disorder frequently have psychiatric co-morbidity, particularly major depression, and biological factors may also explain the association between psoriasis and MDD.18 Patients with IBDs, such as ulcerative colitis and Crohn's disease, frequently have co-morbid depression and related disorders.19 Clinically, significant depression can occur in up to half of all patients with multiple sclerosis and is associated with increased morbidity and mortality. Considering the above findings, we hypothesize that psychopathological factors, as well as biological factors, may explain the association between these autoimmune diseases and MDD.
However, to date, compared to other monocytic, Th1, and Th2 cytokines, the IL-23 and IL-17 axis has been less investigated in the context of mood disorders. We hypothesized that Th17-related pro-inflammatory cytokines have a role in MDD pathogenesis. Our study investigated whether the plasma levels of IL-17 and IL-23 are higher in MDD patients than in normal controls. Further, we investigated the plasma levels of IL-17 and IL-23 after 6 weeks of antidepressant treatment, comparing these with baseline levels in MDD patients.
METHODS
Subjects
Twenty-six MDD patients among the psychiatric patients who were admitted to Korea University Ansan Hospital from May 2007 to December 2010 were recruited. Patients were diagnosed based on the Diagnostic and Statistical Manual of Mental Disorder (DSM-IV) for MDD.20 Patients with co-morbid psychiatric disorders such as bipolar disorder or schizoaffective disorder were excluded. Patients with medical conditions (such as autoimmune or infective disorders) or receiving medications (such as corticosteroids and antibiotics) that could affect plasma cytokine levels were also excluded.
Psychiatric interviews were conducted by a trained physician using the DSM-IV Structured Clinical Interview. Depressive symptoms were assessed using the Hamilton Depression Rating Scale (HDRS)21 at baseline and 6 weeks after treatment. The baseline HDRS scores of MDD patients were ≥18 (27.9±5.1).
MDD patients were treated with various antidepressants. Of the 26 patients, 7 were treated with venlafaxine (mean, 112.5 mg; range, 37.5-150 mg), 6 with paroxetine (mean, 27.08 mg; range, 25-37.5 mg), 5 with escitalopram (mean, 17 mg; range, 10-20 mg), 3 with mirtazapine (mean, 20 mg; range, 15-30 mg), 3 with bupropion (300 mg), and 2 with fluoxetine (20 mg). The mean and standard deviation (SD) of HDRS scores after 6 weeks of antidepressant treatments in the MDD patients was 9.8±8.3.
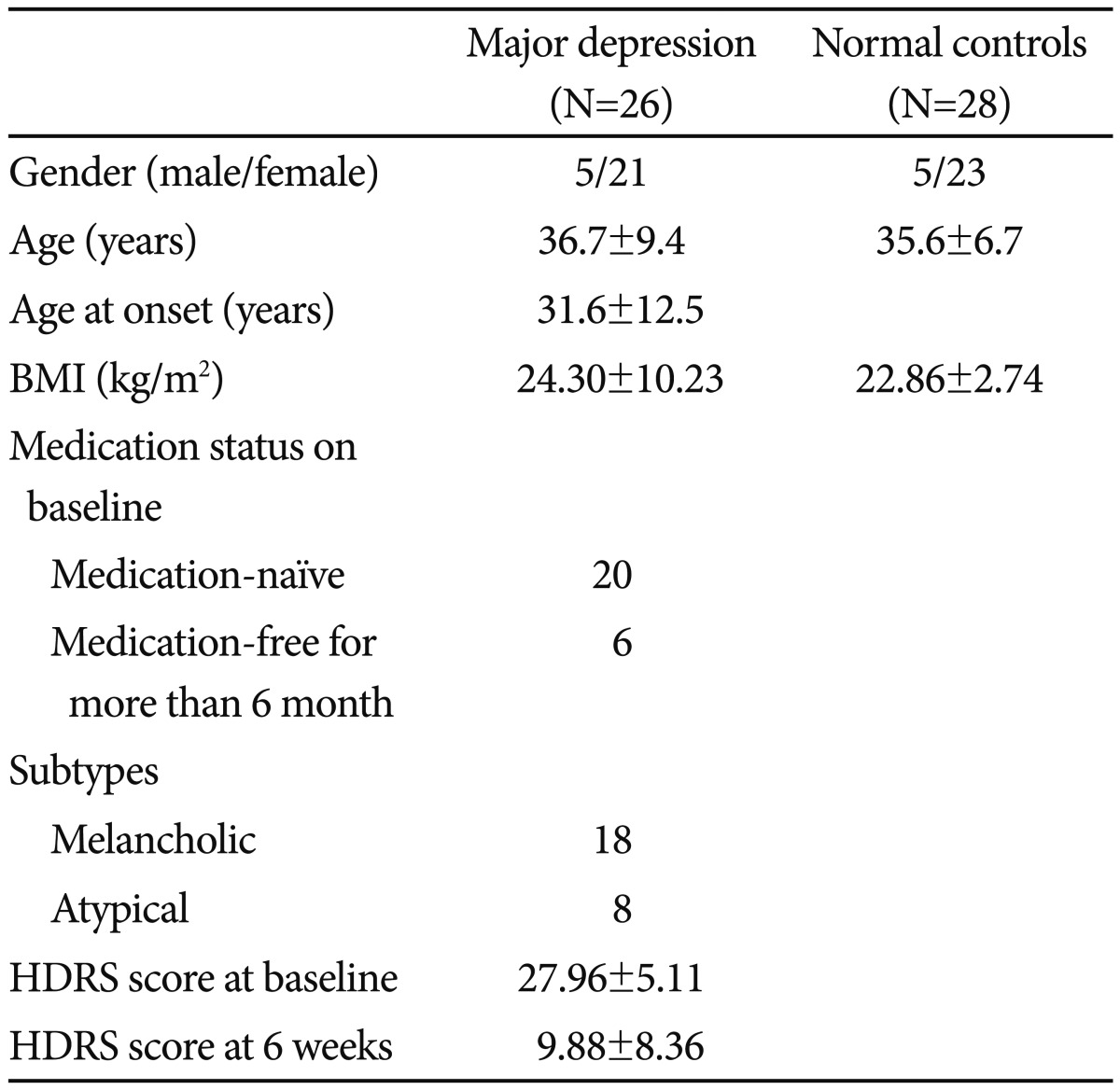
Twenty-eight healthy, non-psychiatric normal controls were recruited through local advertisements and received a monetary reward for their participation. They agreed to participate in the present study and were given a non-structured clinical interview. Subjects who had past psychiatric history, a family history of mental illness among first and second-degree relatives, substance abuse history, autoimmune disease, or infective disorder were excluded. All normal controls had been free from physical illness within the 2 weeks before the study. They showed normal findings from several laboratory tests, including blood chemistry, liver/thyroid function test, chest X-ray radiography, and electrocardiography. Demographic data of patients and normal controls are summarized in Table 1.
Informed consent was obtained from both the groups. This study was approved by the Korea University Ansan Hospital Institutional Review Board.
Sample collection and measurement of plasma levels of IL-17 and IL-23
Ulnar venous blood samples (2 mL) were collected in a lithium heparin vacuum tube for 1 h (from 8:00 A.M. to 9:00 A.M.) for both the groups. After centrifugation at 3000 rpm for 10 min, plasma samples were divided into microcentrifuge tubes (Eppendorf), and frozen immediately at -70℃ until use.
Plasma levels of IL-17 and IL-23 were determined by a solid-phase sandwich enzyme immunoassay using a human IL-17 and IL-23 enzyme-linked immunosorbent assay kit (Wuhan EIAab Science, USCNLIFE, CHINA, cat# E0063h and E0384h). Assays were performed in duplicate according to the manufacturer's recommendations.
Briefly, 100 µL of standards and samples were pipetted into each well and incubated at 37℃ for 2 h. Cytokines were bound by the detection antibody (100 µL of the detection reagents A and B) and incubated at room temperature for 2 h. Following a final washing procedure, 90 µL of substrate solution was added to each well and incubated at 37℃ for 30 min. Subsequently, 50 µL of stop solution was added to each well. The optical density of the color reaction was read using a microplate reader at a wavelength of 450 nm. The intra- and inter-assay coefficients of variation for all analyses were less than 8%.
Statistical analyses
We used the Statistical Package for the Social Sciences (SPSS) version 12.0 for all analyses. Regarding the relatively small sample size, we analyzed our samples by nonparametric method. Mann-Whitney test were used for comparing baseline plasma levels of IL-17, IL-23 of the both groups while Wilcoxon signed rank test examined the changes in cytokine level after antidepressant treatment. Chi-square tests were conducted to analyze the association between the groups for discrete covariates. Correlations between levels of IL-17 and IL-23 and HDRS score were evaluated using the Spearman's correlation test. The null hypothesis was rejected at p<0.05.
RESULTS
Demographic data
The characteristics of the study population are listed in Table 1. There were no significant differences in the male/female ratio (χ2=0.017, p=0.897), age (Z=-0.172, p=0.864), and body mass index (BMI) (Z=-0.912, p=0.362) between the MDD patients and normal controls.
Baseline plasma cytokine levels in MDD patients and normal controls
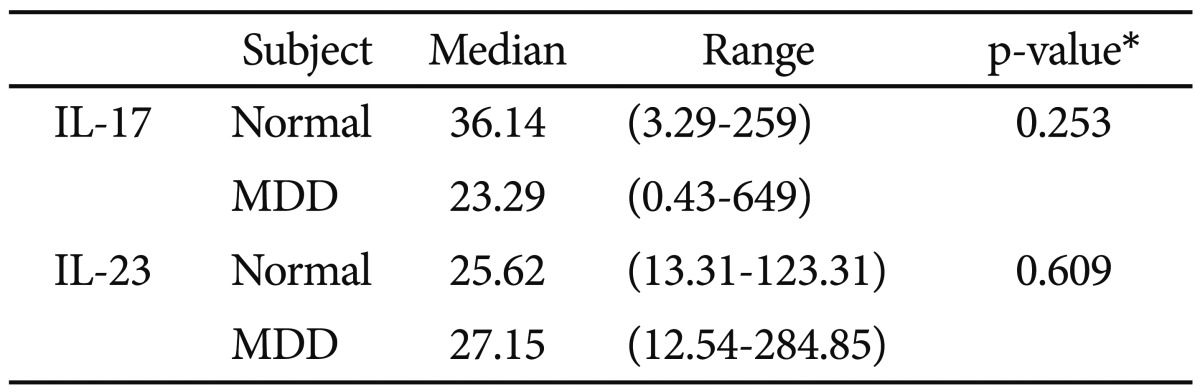
IL-17 and IL-23 was detected in all MDD patients and controls. The baseline plasma levels of IL-17 and IL-23 of MDD patients was not statistically different from those of controls (Table 2, Figure 1).
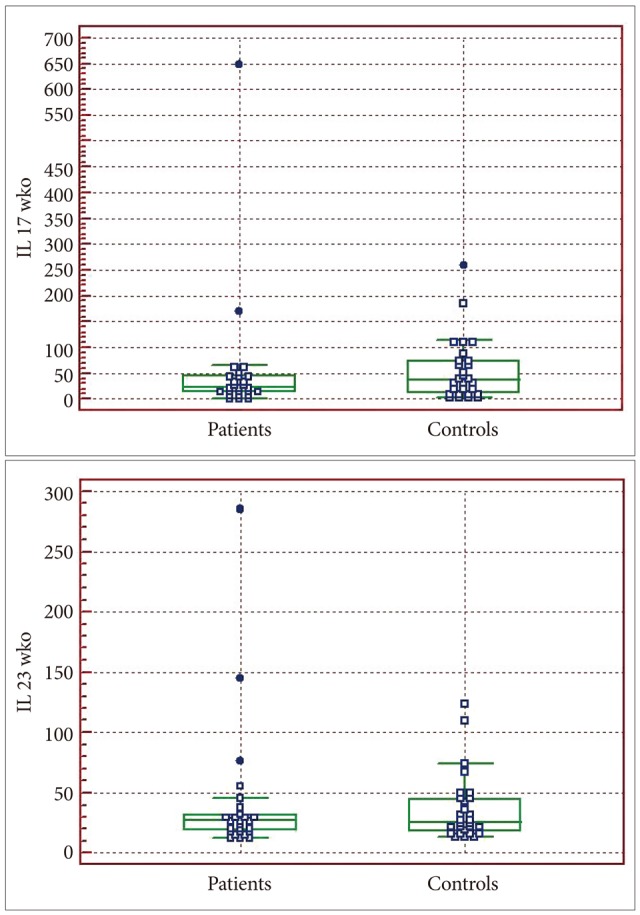
Comparison of the baseline cytokine levels between normal controls and MDD patients. MDD: major depressive disorder.
Further, no statistically significant differences were observed in cytokine levels between the melancholic and atypical subtypes of MDD patients (p=0.657 for IL-17; p=0.824 for IL-23).
Levels of these cytokines and HDRS score of MDD patients were not significantly correlated (rs=-0.133, p=0.517 for IL-17; rs=0.111, p=0.590 for IL-23).
Changes in plasma cytokine levels after antidepressant treatment in MDD patients
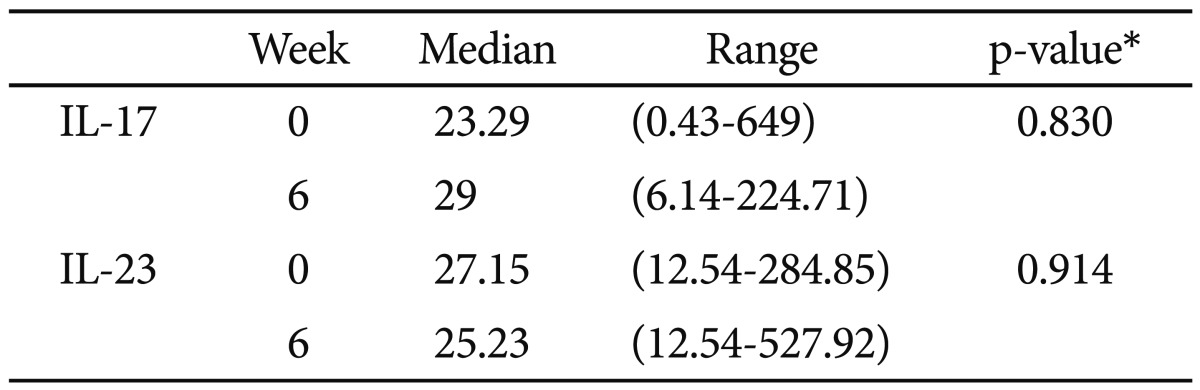
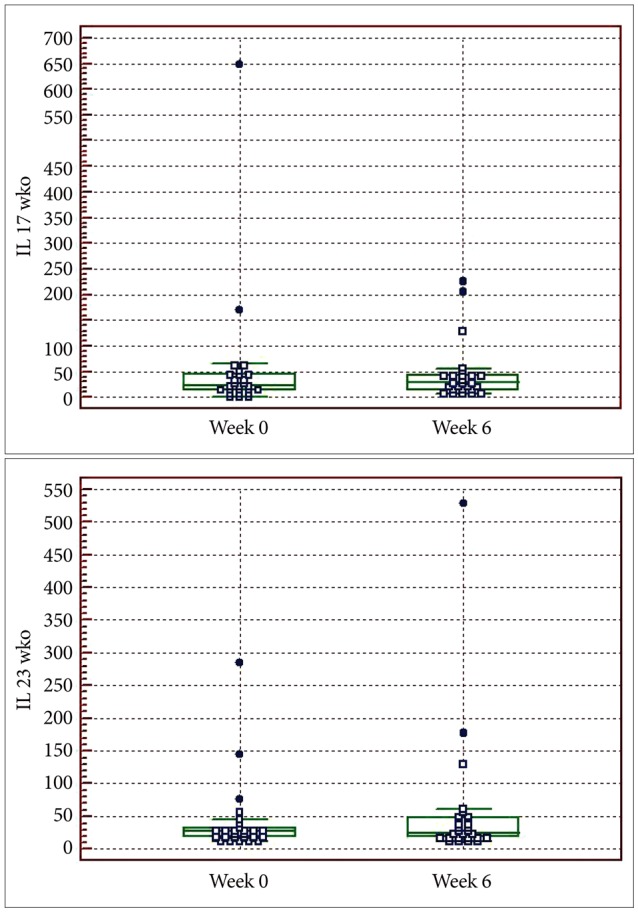
In MDD patients, IL-17 and IL-23 levels did not significantly change after 6 weeks of antidepressant treatments (Table 3, Figure 2). The changes in cytokine levels after 6 weeks of antidepressant treatment and the HDRS score at 6 weeks after treatment were not significantly correlated (rs=0.113, p=0.581 for IL-17; rs=-0.099, p=0.629 for IL-23).
DISCUSSION
The first major finding of our study is that IL-23 and IL-17 levels in MDD patients were not significantly higher than those in normal controls. Another study reported that increased IL-17 levels are associated with the severity of anxiety in rheumatoid arthritis patients.22 These studies indicate a possibility that IL-17, among the cytokines related to Th17 cells, may be associated with anxiety symptoms in autoimmune disease, not with psychiatric diseases. However, Chen et al. reported that a Th17/Treg imbalance exists in MDD patients, which may have implications for the pathogenic role of Th17 cells in MDD.23 There is difference between Chen's study and our study; Chen's study showed relative male preference in gender distribution but our study included relatively more female subjects.
The second major finding of this study is that IL-23 and IL-17 levels in MDD patients did not decreased after 6 weeks of antidepressant treatment. A previous study reported various antidepressants, including tricyclic antidepressants, selective serotonin re-uptake inhibitors (SSRIs), and serotonin-noradrenaline reuptake inhibitors, show negative immunoregulatory effects by suppressing the IFN-/IL-10 production ratio.24 Moreover, many studies reported decreased levels of various proinflammatory cytokines after antidepressant treatment.25-28 However, our findings does not support a potential negative immunoregulatory effects of IL-23 and IL-17 axis after antidepressants treatment.
Recently, the cytokine hypothesis of depression has received attention for several reasons. First, chronic inflammatory diseases are frequently associated with depression. Second, the association between immune dysfunction and MDD is bidirectional. Not only are serum levels of many pro-inflammatory cytokines increased in depressive disorder, but also the medications that affect the cytokine system affect mood. Endotoxin administration in humans results in sickness behavior, whereas endotoxin has little effects on physical sickness symptoms.29 Third, these sickness behaviors improve when treated with antidepressants.30 Postmortem studies of brain tissues from drug-naïve MDD patients have shown increased expression of pro-inflammatory cytokines.31
Although the majority of studies support the cytokine hypothesis of MDD which implies positive association between immune dysregulation and MDD, there also have been some reports against this theory. There have been studies of outpatients with depressive disorder that showed no significant differences in pro-inflammatory cytokine profiles when compared to normal controls.32-34 If immune dysregulation causes elevation of cytokine levels in MDD, remission or even a reduction in depressive symptoms would be expected to reduce the cytokine levels. However, Hannestad et al. reported that a meta-analysis of various studies failed to show a statistically significant effect of antidepressant treatment on serum levels of IL-6 and TNF-α.35 Another group of studies reported that SSRI treatment did not significantly change TNF-α serum levels regardless of the reduction in depressive symptoms.36,37 Our results conflict the cytokine theory of major depression. In our study, the HDRS score significantly reduced after 6 weeks of antidepressant treatment (27.96±5.11 to 9.88±8.36). However, no significant differences in cytokine levels were observed, despite the marked improvement of depressive symptoms.
Our results have some limitations. First, the relatively small sample size may have limited the power of a possible association between the IL-23/IL-17 axis and major depression. Second, although the normal controls underwent a clinical interview, subclinical depressive symptoms might not be detected. Psychological stresses may affect the cytokine profiles of normal controls, resulting in increased pro-inflammatory cytokine levels.
However, in addition to the limitations, our study has various strengths. Numerous confounding factors that can affect the plasma levels of cytokines, such as gender, age, and BMI,38 were controlled. We tried to exclude the effect of drug use. Twenty patients were medication-naïve and another 6 patients were enrolled after a medication-free period of at least 6 months. Patients with medication-free period of less than 6 months were excluded because use of antidepressants can affect serum cytokine levels. Further, we tried to minimize the difference of age and BMI between the MDD patients and control groups; no statistically significant differences were found in age and BMI. Although we collected samples from groups with a similar male/female ratio, we could not explore the effect of gender on cytokines due to the preponderance of females (5 males and 21 females in MDD and 5 males and 23 females in controls). This factor should be focused on in future studies.
In conclusion, we failed to find a statistically significant association between the IL-23/IL-17 axis and MDD. However, there have been some reports suggesting a pathogenic role of Th17 cells in MDD. Future studies with larger sample sizes are necessary to confirm our results.
Acknowledgments
This paper was done as a part of master's thesis (in Medicine) of Dr. Kim J-W.