Prevalence of Metabolic Syndrome in Patients with Schizophrenia in Korea: A Multicenter Nationwide Cross-Sectional Study
Article information
Abstract
Objective
We designed a nationwide study with limited exclusion criteria to investigate the prevalence of metabolic syndrome (MetS) in Korea and its relationship with antipsychotic medications.
Methods
This multicenter, cross-sectional, and observational study included patients diagnosed with schizophrenia or schizoaffective disorder. Sixteen hospitals enrolled 845 patients aged 18 to 65 years prescribed any antipsychotic medication between August 2011 and August 2013. MetS was diagnosed using the criteria of the modified Adult Treatment Panel III of the National Cholesterol Education Program with the Korean abdominal obesity definition (waist circumference ≥85 cm in women, ≥90 cm in men).
Results
The prevalence of MetS in all patients was 36.5% and was significantly higher in men than women (men, 40.8%; women, 32.2%) and was significantly correlated with age [odds ratio (OR) 1.02] and duration of illness (OR 1.03). The prevalence of MetS across antipsychotic drugs in the major monotherapy group was as follows: 18.8% for quetiapine, 22.0% for aripiprazole, 33.3% for both amisulpride and paliperidone, 34.0% for olanzapine, 35% for risperidone, 39.4% for haloperidol, and 44.7% for clozapine.
Conclusion
The prevalence of MetS is very high in patients with schizophrenia or schizoaffective disorder. Screening and monitoring of MetS is also strongly recommended.
INTRODUCTION
Patients with schizophrenia have a reduced life expectancy for several reasons, including increased incidence of accidents, cardiovascular disease (CVD), and infectious disease.1 In particular, CVD is one of the most important causes of mortality and is responsible for about 45% of the excess mortality of schizophrenia.2 Patients with schizophrenia show 2−3 times higher risk of CVD than healthy controls.1
Many factors, including poor diet, tobacco smoking, weight gain, and low physical exercise, affect CVD development in patients with schizophrenia.345 Recently, several medications, especially antipsychotics, have been identified as having a negative impact on the development of CVD.67 Several comprehensive reviews concluded that atypical antipsychotics significantly increased the risk of certain CVDs, such as diabetes, compared with typical antipsychotics.68 Some studies also reported a higher prevalence of diabetes and hypertension in patients with schizophrenia than healthy controls.3910
This increased prevalence of CVD, diabetes, and hypertension and increased mortality may be partially due to Metabolic Syndrome (MetS). MetS includes five major components: 1) abdominal obesity; 2) hypertension; 3) increased fasting glucose level; 4) hypertriglyceridemia; and 5) decreased high-density lipoprotein (HDL).
The prevalence of MetS in patients with schizophrenia is 2−4 times higher than in healthy individuals.8111213 The prevalence of MetS in patients with schizophrenia varies across studies: 35.4% in the Netherlands,14 32.3% in Belgium,15 40.9% in the United States CATIE study,12 22.8% in Thailand,16 27.5% in Japan,17 and 31.7% in Korea.13
The causes of this variety in the prevalence include the use of different definitions of MetS, such as that of the Adult Treatment Panel III report (ATP III) of the National Cholesterol Education Program,1214 the modified ATP III,131517 and the International Diabetes Federation (IDF).16 In addition, some studies of Asian patients used Asian or Korean criteria for abdominal obesity. MetS is also partially dependent on the location, lifestyle, and demographic characteristics of the patients, such as age, gender, and ethnicity.181920 Patients in previous studies had very different demographic and clinical factors, such as mean age, male:female ratio, duration of illness, and prescribed antipsychotic medications. Finally, some studies investigated the prevalence of MetS in a limited number of patients or patients enrolled for other purposes and not for the actual study of this prevalence. Thus, there may have been a selection bias and the results from a limited number of patients or hospitals might not be generalizable.
To overcome these limitations, we designed a nationwide study with minimal exclusion criteria and from multiple psychiatric units across the nation to investigate the prevalence of MetS in Korea and the relationship between psychiatric medication and MetS prevalence.
METHODS
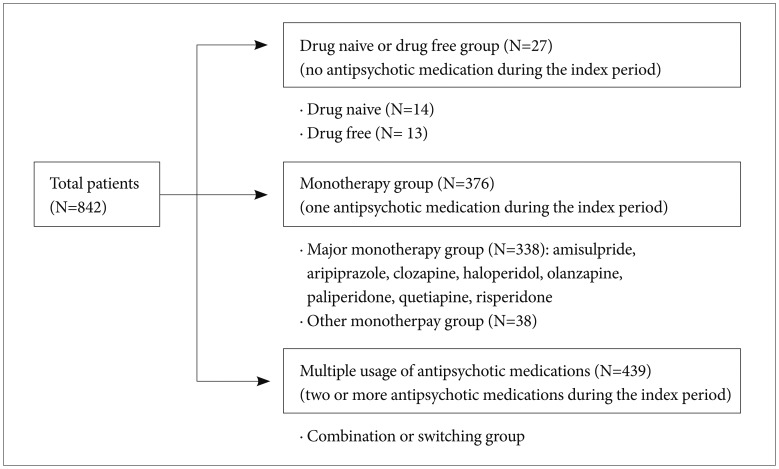
This study was a multicenter, cross-sectional, and observational study of patients diagnosed with schizophrenia or schizoaffective disorder according to Diagnostic and Statistical Manual of Mental Disorders-IV-Text Revision (DSM-IV-TR) diagnostic criteria. Sixteen hospitals−3 mental hospitals and 13 university-affiliated general hospitals−enrolled patients aged 18 to 65 years that had been prescribed any antipsychotic medication for the treatment of schizophrenia or schizoaffective disorder between August 2011 and August 2013. Of the 892 patients who consented to participate in the study, we excluded 47 due to a lack of information, such as age, gender, blood pressure (BP), waist circumference, or blood laboratory results. Written informed consent was obtained from all patients and this study was approved by the Institutional Review Board of each hospital.
Patient demographic data and histories were obtained from their medical records and by interview. We asked the patients if they had any conditions such as diabetes, dyslipidemia, or hypertension, or were overweight when they enrolled in this study. Based on the patients' reports, we divided these subjects into “patients with no medical disease” and “patients with medical disease”. Waist circumference was measured at the umbilical level in a standing position and BP was measured using standard mercury sphygmomanometers or clinically approved automatic BP devices after a 5-minute rest. Plasma HDL cholesterol, triglycerides, and fasting blood glucose were also measured after at least 8-hour fast. MetS was diagnosed with the definitions of the modified ATP III for Korea (three or more of the following five criteria):21 waist circumference ≥85 cm in women or ≥90 in men, fasting blood glucose ≥100 mg/dL or specific treatment for hyperglycemia, serum triglyceride ≥150 mg/dL or specific treatment for a lipid abnormality, HDL <40 mg/dL in men or <50 mg/dL in women or specific treatment for a lipid abnormality, and arterial BP (≥130/85) or specific treatment for hypertension.
We defined the index date of each patient as the date of blood sampling and the index period as the 12-month period before the index date. We collected information on the dosage and duration of antipsychotics prescribed during the index period. We defined the “monotherapy group” as those patients who took only one antipsychotic drug during the index period. In the monotherapy group, patients were defined as the “major monotherapy group” if they took one of the following antipsychotics: amisulpride, aripiprazole, clozapine, haloperidol, olanzapine, paliperidone, quetiapine, and risperidone.
To investigate the relationship between antipsychotic medication and MetS, we compared the prevalence of MetS in the major monotherapy group according to the antipsychotic medications. In addition, to estimate the effect of the antipsychotic dose on the development of MetS in the major monotherapy group, the doses of the major antipsychotics were converted to the olanzapine equivalent dose. We defined the cumulative equivalent dose as the sum of the equivalent doses of the major antipsychotics prescribed to the patient during the index period. We then investigated the effect of the cumulative equivalent dose and mean equivalent dose on the development of MetS in the major monotherapy group. Equivalent ratios of antipsychotics to olanzapine 1 mg were as follows: 0.029 mg for amisulpride, 0.67 mg for aripiprazole, 0.05 mg for clozapine, 2 mg for haloperidol, 2.22 mg for paliperidone, 0.027 mg for quetiapine, and 3.33 mg for risperidone.22
All statistical analyses were performed using STATA (ver. 13; Stata Corp., TX, USA). Comparisons of the prevalence of MetS according to antipsychotic medications or gender were made with the chi-square test. Correlations between MetS and other clinical variables such as age and duration of illness were performed using logistic regression analysis. All statistical analyses used two-tailed test and p value <0.05 was considered to be statistically significant.
RESULTS
Demographic data and clinical characteristics
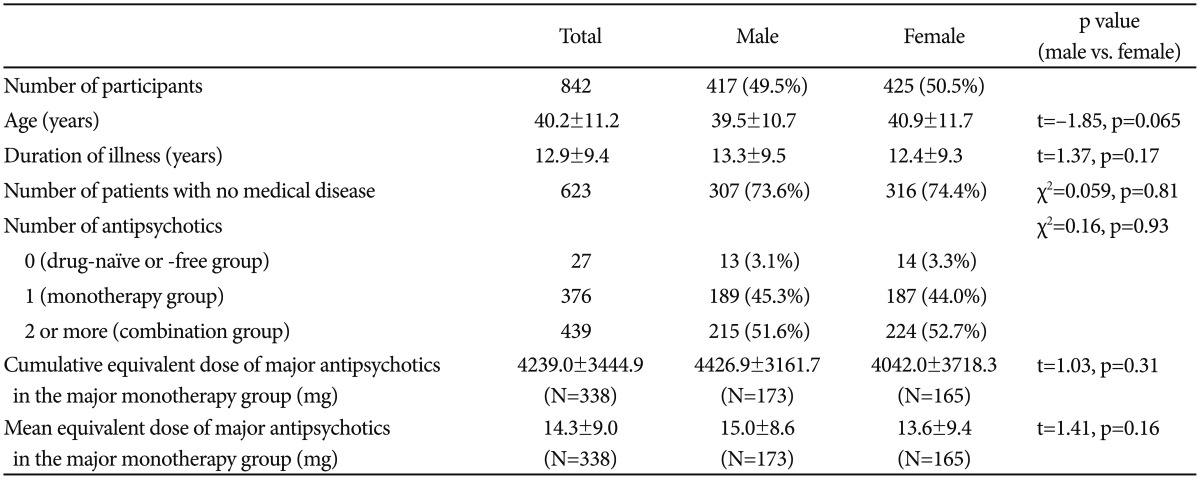
The number of patients in the final analysis was 842. The demographic and clinical characteristics are presented in Table 1. The mean patient age was 40.2±11.2 years (range 18−65) and the number of male patients was 417 (49.5%). There was no significant difference in age according to gender (men, 39.5±10.7; women, 40.9±11.7, t=-1.85, p=0.065). The mean illness duration in all participants was 12.9±9.4 years (range 0−44). About 26% patients (n=219) reported that they had one or more medical diseases related to MetS when they enrolled in this study (defined as the medical disease group).
Clinical and Demographic characteristics of participants with schizophrenia or schizoaffective disorder
Of the 842 enrolled patients, 27 did not take any antipsychotics during the index period; 14 were drug-naïve patients and the other patients (n=13) took antipsychotics more than 1 year before the index date. In addition, 376 patients (monotherapy group) took only one of 17 antipsychotic medications during the index period. The numbers of patients in the major monotherapy group by the antipsychotics were as follows: risperidone (n=100), clozapine (n=46), olanzapine (n=46), aripiprazole (n=41), paliperidone (n=39), haloperidol (n=32), amisulpride (n=18), and quetiapine (n=16). The other patients (n=38) took one of nine antipsychotics (Figure 1, Table 1).
There were no significant differences in the cumulative and mean equivalent doses of antipsychotics in the major monotherapy group between men and women (cumulative equivalent dose: 4426.9±3161.7 mg vs. 3042.1±3718.3 mg, t=1.03, p=0.31; mean equivalent dose: 15.0±8.6 mg vs. 13.6±9.4 mg, t=1.4, p=0.16) (Table 1).
Prevalence of MetS
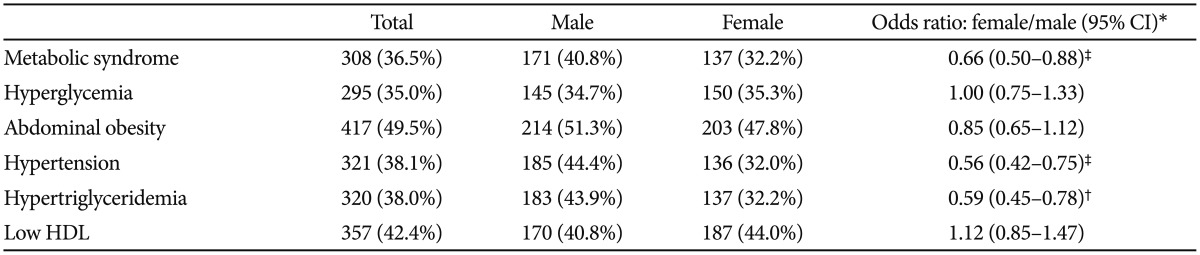
The prevalence of MetS in all patients was 36.5% (n=307) and was significantly lower in female patients (women, 32.2%; men, 40.8%; OR 0.66, 95% CI 0.50−0.88; age-adjusted p<0.001) and significantly correlated with age (OR 1.02, 95% CI 1.01−1.04; p<0.001) and duration of illness (OR 1.02, 95% CI 1.01−1.04; p=0.002). The prevalence of each component of MetS ranged from 35% to 50% (hyperglycemia, 35.0%; hypertriglyceridemia, 38.0%; HTN, 38.1%; low HDL, 42.4%; abdominal obesity, 49.5%). Abdominal obesity was the most common component for both sexes (men, 51.3%; women, 47.8%). There were significant differences in the prevalence of hypertension (men, 44.4%; women, 32.0%; OR 0.56, 95% CI 0.42−0.75; age-adjusted p<0.001) and hypertriglyceridemia (men, 43.9%; women, 32.2%; OR 0.59, 95% CI 0.45−0.78; age-adjusted p=0.009) by sex (Table 2).
Although 176 (28.3%) of the 623 patients with no medical disease were diagnosed with MetS, 131 (59.8%) of the 219 patients who reported they had one or more MetS-related medical diseases were diagnosed with MetS.
Relationship between antipsychotic medications and the prevalence of MetS
The prevalence of MetS was slightly different by the number of antipsychotic medications but it was not statistically significant (df=2,839, F=0.2, p=0.762): 33.3% (drug-naïve or free group), 35.4% (monotherapy group), 37.6% (multiple medication group).
Because of the possibility of a different effect of the antipsychotics on MetS, we used only data in the major monotherapy group to investigate the relationship between antipsychotics and MetS. In the major monotherapy group (n=338), the prevalence of MetS according to antipsychotic medications was as follows: 18.8% for quetiapine, 22.0% for aripiprazole, 33.3% for both amisulpride and paliperidone, 34.0% for olanzapine, 35% for risperidone, 39.4% for haloperidol, and 44.7% for clozapine (Figure 2). The difference of the prevalence of MetS between quetiapine group and aripiprazole group was not significant (χ2=0.07, p=0.790). The prevalence of MetS strongly increased with an increase in the cumulative equivalent dose of the seven atypical antipsychotics in the major monotherapy group (OR 1.00012, 95% CI 1.000032−1.000204, age- and sex-adjusted p=0.007). However, one typical antipsychotic drug (haloperidol) did not show this significant correlation (OR 0.99989, 95% CI 0.99975−1.00004, age- and sex-adjusted p=0.16).
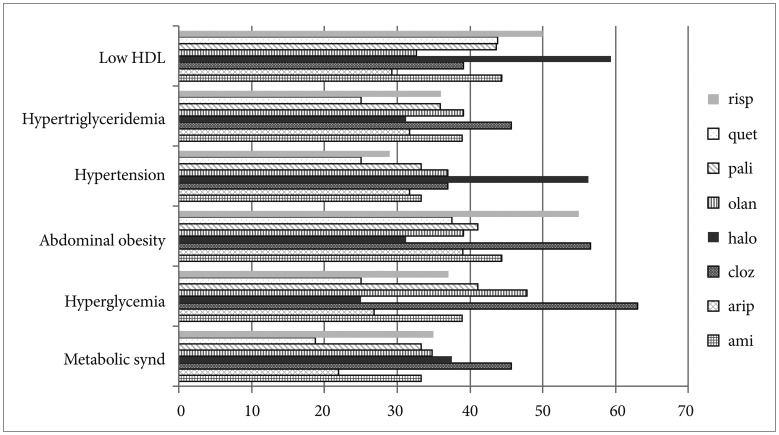
Prevalence of metabolic syndrome and its five components across the major antipsychotics administered during the index period. risp: risperidone, quet: quetiapine, pali: paliperidone, olan: olanzapine, halo: haloperidol, cloz: clozapine, arip: aripiprazole, ami: amisulpride, HDL: high-density lipoprotein.
There were no significant correlations found between the development of MetS and the mean equivalent dose of both the seven atypical antipsychotics (OR 1.03, 95% CI 1.00−1.06, age- and sex-adjusted p=0.069) and the one typical antipsychotic drug (OR 0.95, 95% CI 0.90−1.01, age- and sex-adjusted p=0.083).
DISCUSSION
Our present study showed an overall prevalence of MetS in patients with schizophrenia or schizoaffective disorder of 36.5%, with a higher prevalence in male patients (40.8% vs. 32.2%). In addition, we found that the prevalence of MetS increased significantly with an increase in patient age and duration of illness. When we analyzed the relationship between antipsychotics and MetS in patients prescribed major antipsychotics in the monotherapy group, the prevalence of MetS was lowest with quetiapine (18.8%) and aripiprazole (22.0%) and highest with haloperidol (37.5%) and clozapine (45.7%). A significant correlation between the cumulative 1-year dose and the development of MetS was found in patients prescribed one of the seven atypical major antipsychotics, not in patients prescribed a typical antipsychotic drug.
The overall prevalence (36.5%) of MetS in our current study was higher than that reported in other studies with Asian including reports from Thailand (22.8%), Taiwan (34.9% and 23.8%), Japan (27.5%), and Korea (31.7%)1316172324 and was more comparable to the rates reported from western countries,12 although most Asian studies used a lower criterion (≥80 cm) of abdominal obesity than we did (≥85 cm). It is difficult to identify the reasons for this discrepancy because many factors affect the development of MetS, including medication, age, gender, definition of MetS, and lifestyle. However, one of the important causes may be the trend for an increase in obesity and MetS during the past two decades in the Korean healthy population. A population-based study (n=183,159) has reported that the prevalence of obesity in children and adolescents in Korea increased from 5.8% in 1997 to 9.7% in 2005.25 In addition, according to the population-based surveys performed in 1998, 2001, 2005, and 2007 in Korea, the age-adjusted prevalence of MetS increased significantly from 24.9% in 1998 to 29.2% in 2001, 30.4% in 2005, and 31.3% in 2007.26 Given this trend in healthy people, the results of our study, which enrolled patients from 2011 to 2013, can be more readily understood.
Our study found that the prevalence of MetS in male patients was eight percentage points higher than that of female patients. Other studies typically showed a higher prevalence of MetS in female patients.122327 However, some studies of Asian patients using the modified ATP III criteria also showed a 2.6−8% higher prevalence of MetS in men.172324 This male preponderance of MetS observed in our study and other Asian reports can be explained by the use of different definitions of abdominal obesity. According to Tan et al.,28 the decreased waist circumference of the modified ATP III for the Asian healthy population increased the crude prevalence of MetS. This effect was more prominent in men (7.8% increase) than in women (4.5% increase). In addition, the use of a stricter definition of abdominal obesity for women (≥85 cm) in our current study than the Asian criterion (≥80 cm) could explain the higher prevalence of MetS in male patients.
Patients with schizophrenia or schizoaffective disorder have several risk factors for MetS. First, smoking is a well-known risk factor for this condition.29 Patients with schizophrenia have a higher frequency of smoking than healthy controls or nonpsychotic patients.13031 Second, patients with schizophrenia tend to perform little exercise and eat more fat or sugar.532 These unhealthy lifestyle behaviors can play a role in MetS development in patients with schizophrenia. Third, many studies reported that some atypical antipsychotics, especially olanzapine and clozapine, were associated with an increase in diabetes, weight gain, and MetS.33343536 According to a previous meta-analysis of head-to-head comparisons of the metabolic side effects of atypical antipsychotics,37 olanzapine produces more weight gain and a greater increase in cholesterol and glucose than other atypical antipsychotics. However, aripiprazole among atypical antipsychotics has shown a relatively lower risk of MetS than other antipsychotics.383940 In line with these reports, the prevalence of MetS was highest in our clozapine group and the patients who had been prescribed aripiprazole had the second lowest prevalence of MetS. Although the lowest prevalence was in the quetiapine group in our study, the size of this group was too small (n=16) to generalize its results.
We found a significant correlation between the cumulative equivalent dose and the increased prevalence of MetS in patients prescribed one of seven major atypical antipsychotics, and not in those prescribed a typical antipsychotic drug. However, this finding should be cautiously interpreted because we used only drug information during the 1 year before the index date for analysis and we did not obtain other information on risk factors such as lifestyle. Because MetS occurs insidiously and there are many risk factors of difficult quantification, it is difficult to identify the relationship between the antipsychotic dose and MetS. A literature review has reported a dose-dependent relationship between the metabolic outcomes and serum concentrations only, not the daily administered dose of olanzapine and clozapine.41 Osborn et al.42 reported a positive correlation between the antipsychotic dosage and cardiovascular mortality. Short-term studies have also reported increased weight gain, fasting glucose, and body fat during short-term atypical antipsychotic treatment.4344
Some patients in our study took anti-depressant or mood stabilizers longer than 30 days during the index period (respectively, 157 and 149 patients). These medications have variable and inconsistent effects on MetS. Although most SSRI except paroxetine have a neutral effect on the development of the side effects related to MetS, paroxetine, venlafaxine, and mirtazapine were associated with the increase of these side effects.45 Bupropion is related to the decreased weight.46 Our study showed no significant difference of the rate of MetS between the patients who took anti-depressant longer than 30 days and the patients who did not (respectively, 31.8% vs. 37.5%, df=1,840, F=1.77, p=0.183). Regarding to mood stabilizers, topiramate was related to the decreased weight,4748 while valproate, in contrast, was associated with increased weight gain.49 We could not find the significant effect of mood stabilizers on MetS by comparison between the patients who took mood stabilizers longer than 30 days and the patients who did not (prevalence of Mets: 36.2% vs. 36.5%, respectively, df=1,840, F=0.00, p=0.951).
Our study had several limitations. First, although we tried to enroll patients who would be representative of the entire Korean population with schizophrenia or schizoaffective disorder, there was the possibility of a selection bias due to the requirement for written informed consent. However, we estimated the prevalence of MetS from data on 842 patients, which is about 0.74% of the estimated 114,222 total number of schizophrenia patients in Korea according to a 2011 nationwide epidemiological survey of mental disorders.50 Second, we cannot conclude that our findings on the effects of antipsychotic medication on MetS indicate causality because we could not obtain complete information on the antipsychotic medication prescribed to patients after onset and psychiatrists might have prescribed low-risk medications to patients at high risk of MetS development. Finally, information on prescribed medications for dyslipidemia, hypertension, diabetes, or obesity were not precisely gathered.
Notwithstanding these limitations, our current study has confirmed the high prevalence of MetS in patients with schizophrenia or schizoaffective disorder and that this prevalence differs among antipsychotic medications. In addition, most patients diagnosed with MetS in our study cohort did not know they had it before participation. Therefore, patients who are prescribed antipsychotics should be routinely screened.
Acknowledgments
This study was supported by Korea Otsuka Pharmaceuticals.