Effect of Initial Ziprasidone Dose on Treatment Outcome of Korean Patients with Acute Manic or Mixed Episodes
Article information
Abstract
Objective
We investigated the efficacy and tolerability of ziprasidone combined with divalproex to determine the relationship between the initial dose of ziprasidone and the treatment effect among Korean patients with acute bipolar manic or mixed disorders.
Methods
This study was a 6-week, open-label, prospective investigation of Korean patients with an acute manic or mixed episode of bipolar disorder. Sixty-five patients were recruited. The patients were categorized based on the initial dose of ziprasidone as follows: low (20-79 mg/day) and standard (80 mg/day). Ziprasidone was given in combination with divalproex in flexible doses, according to the clinical response and tolerability.
Results
The response and remission rates were significantly higher in the standard-dose group than the low-dose group. The combination of ziprasidone and divalproex was well-tolerated and adverse events were mostly mild with no statistically significant increase in body weight.
Conclusion
The results of this study showed that a standard starting dose of ziprasidone in combination with divalproex for bipolar disorder is more effective than a low starting dose.
INTRODUCTION
Dosing recommendations contained in product labeling may differ from dosing implemented in clinical practice because the recommendations reflect the design and results of registration studies. These studies may be designed without the benefit of complete knowledge of dose-response relationships, and implemented in study subjects who may differ from the patients who will actually receive these medications.1 After gaining clinical experience with new medications, clinicians may modify their prescribing practices, with some anti-psychotics being prescribed at much lower or much higher doses than originally anticipated, such as has been reported for risperidone, olanzapine, and quetiapine in hospitalized patients.2
Ziprasidone is a novel anti-psychotic with a unique pattern of receptor affinity that distinguishes it from other anti-psychotics. Ziprasidone is a potent antagonist at the serotonin 5-HT2A, 5-HT1D, 5-HT2C receptors, and dopamine D2 receptor with a high 5-HT2A/D2 ratio, and a potent agonist at 5-HT1A receptors.3 In randomized, placebo-controlled studies involving patients with acute bipolar manic or mixed episodes, ziprasidone monotherapy was shown to produce rapid, sustained improvements in manic and psychotic symptoms relative to the baseline and placebo at study end points, and demonstrated a favorable safety and tolerability profile.4,5
The ziprasidone product label6 recommends an initial daily dose of 80 mg/d, with an increase to 120 or 160 mg/d on the 2nd day of treatment for patients with bipolar mania. A recent analysis of data from a wide range of fixed- and flexible-dose trials showed that doses of ziprasidone between 120 and 160 mg/day were associated with superior and more rapid improvement in overall psychopathology than lower doses of this medication.7,8 These study results also revealed that ziprasidone doses >120 mg were associated with lower rates of early discontinuation due to inadequate clinical responses within 14 days of treatment, as compared to lower doses. Furthermore, in placebo-controlled trials, ziprasidone (120-160 mg/day) was associated with a low rate of discontinuation due to adverse events.9,10
Although the optimal dose of ziprasidone in controlled clinical trial settings for schizophrenia has been well-documented, there is little evidence in support of the proper dose of ziprasidone in patients with bipolar disorder, especially when combined with a mood stabilizer. Moreover, East Asian patients receive relatively lower doses of anti-psychotics than their Caucasian counterparts.11-13
Such findings suggest the need for further evaluation of the relationship between ziprasidone dosing and effectiveness in an Asian 'real world' clinical practice.
The objective of this study was to determine the relationship between initial ziprasidone dose and efficacy, and the safety of a ziprasidone and divalproex combination in the treatment of patients with bipolar manic or mixed episodes.
METHODS
Participants
The present study was an open-label, multi-center, 6-week, flexible-dose study involving combination ziprasidone and divalproex for the acute treatment of patients with bipolar manic or mixed episodes. Patients who fulfilled the inclusion and exclusion criteria listed below were eligible for participation in this trial. Patients had to be at least 18 years of age at the time they enrolled in the study.
The inclusion criteria included a current DSM-IV14 diagnosis of bipolar disorder with a current manic or mixed episode and a requirement for anti-psychotic treatment on the basis of clinical experience or investigator preference. At the time of entry into the study, the patients had to have a score >20 on the Young Mania Rating Scale (YMRS).15 The exclusion criteria were as follows: confirmed organic brain disease, including a history of cerebrovascular accidents, brain tumors, or mental retardation; co-existing severe medical conditions; a history of substance abuse or dependence within 1 month before study entry; the presence of any other axis I DSM-IV diagnoses; the use of depot anti-psychotics within one cycle before study entry; and women who were pregnant or breastfeeding.
All patients were recruited during between May 2007 and December 2007 from 9 nationwide sites in Korea, including university-based hospitals or chronic mental institutions.
Treatment and study procedures
The study subjects were treated with ziprasidone in combination with divalproex for up to 6 weeks. A washout period of 3 days for patients who had received any disallowed concomitant medications, such as anti-psychotics other than ziprasidone or mood stabilizers other than divalproex, preceded study entry.
The study used a flexible dosing schedule for ziprasidone and divalproex. Patients received 40-80 mg/day of ziprasidone on days 1 and 2. After day 3, the dose could be maintained, reduced, or increased up to 160 mg/day, based on the investigator's experience and patient response. Divalproex was started at 500 mg/day and increased to 1,000 mg/day on day 2. The divalproex dose could be maintained or changed within the usual therapeutic dose range. Investigators were instructed to adjust the doses of the divalproex to obtain serum concentrations in the usual therapeutic range (50-120 µg/mL). Anti-psychotics other than ziprasidone, and mood stabilizers other than divalproex, were not allowed during the trial. Lorazepam for agitation (up to 4 mg/day) and anti-Parkinsonian medications were allowed, but preventive use was prohibited.
This study was performed by investigators who had previous experience with clinical trials and the management of patients with bipolar disorder. The investigators were all given training for the instruments used to assess the efficacy of the trial. Prior to beginning the trial, an introductory workshop on the study design and methodology for the study was held in each district.
Outcome assessments
The measures used to assess the efficacy of medication included the YMRS, the 17-item Hamilton Rating Scale for Depression (HAMD),16 the Brief Psychiatric Rating Scale (BPRS),17 and the Clinical Global Impression-Severity (CGI-S).18 The YMRS, HAMD, BPRS, and CGI-S scores were assessed at baseline and at weeks 1, 3, and 6 after treatment commenced.
The Extrapyramidal Symptom Rating Scale (ESRS)19 was administered at each visit in order to assess tolerability. The vital signs and weight were recorded at each visit. All adverse events attributable to or suspected to be related to the study drug were recorded at baseline and at each visit. The patients were categorized into two groups by the initial dose of ziprasidone.20 The patients who were started on <80 mg/day were included in the low-dose start group, and the patients started on 80 mg/day were included in the standard-dose start group.
Statistical analysis
The data included all patients who provided both a baseline and at least 1 post-baseline data measurement. All patients who received at least one dose of the study medication were included in the safety analysis. Comparisons of the baseline demographic and illness characteristics between the low-and standard-dose start groups were performed using an independent t-test for continuous variables and a chi-square test or Fisher's exact test for categorical variables. The mean doses of ziprasidone and divalproex were compared between the low- and standard-dose start groups using an independent t-test. The primary efficacy measure was the mean change in the YMRS total score from baseline to end-point. Last-observation-carried-forward (LOCF) analyses of change from baseline to endpoint in YMRS, HAMD, BPRS, and CGI-S scores were performed. Paired t-tests and repeated measures analysis of covariance (ANCOVA) were used to determine whether there were significant changes in each efficacy variable over time. ANCOVA was conducted with baseline measures, and concomitant medications were entered as covariates. ANCOVA models included the effects of treatment, low-dose start/standard-dose start categorization, and interactions of treatment. In addition, a responder and remitter analysis was performed on the YMRS scores comparing the rate of response and remission among groups by chi-square analysis. A response was categorically defined as a >50% reduction on the YMRS scales. Remission was defined as a total YMRS score of <12. We also performed logistic regression using these variables as predictors and the response and remission as dependent variables. To compare the risk of adverse events between the initial dose groups, chi-square tests were performed. All statistical tests were two-tailed with a significance level of 0.05. Statistical analysis was carried out using SAS, PC version 6.0.
Ethics
The study was conducted according to the Declaration of Helsinki and Good Clinical Practices. Written informed consent was obtained from all subjects after they were given an extensive explanation of the nature and procedures of the study, and patient anonymity should be preserved. The study protocol was approved by the Institutional Review or Ethics Committees at each study site.
RESULTS
Patients
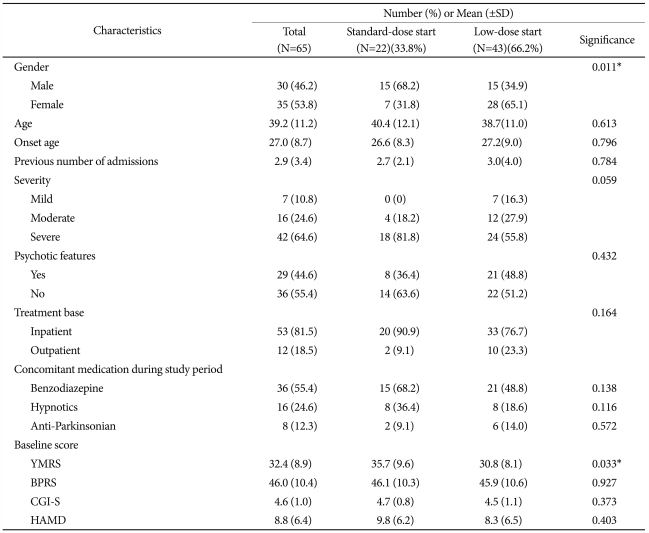
Sixty-five subjects participated in this study. Forty-three patients (66.2%) were included in the low-dose start group and 22 patients (33.8%) were included in the standard-dose start group. In low-dose start group, most of patients (36/43, 83.7%) started ziprasidone on 40 mg/day. The baseline demographic and illness characteristics of the patients in the low-and standard-dose start groups are summarized in Table 1. The baseline YMRS score was significantly higher (p=0.033) in the standard-dose start group (35.7±9.6) than the low-dose start group (30.8±8.1). The other characteristics were similar between the low- and standard-dose start groups. Twenty-one of the 65 patients (32.3%) did not complete the study. The reasons for non-completion were loss to follow-up (n=10, 15.4%), withdrawal of consent (n=5, 7.7%), an adverse event (n=3, 4.6%), lack of efficacy (n=1, 1.5%), non-compliance (n=1, 1.5%), and protocol violation (n=1, 1.5%); 17 patients (81.0%) were in low-dose start group and 4 patients (19.0%) were in standard-dose start group (p=0.082).
Medications
The mean doses of ziprasidone and divalproex used in the current study are presented in Table 2. There was no difference between the low- and standard-dose start groups with respect to the mean doses of ziprasidone (95.0±29.3 mg/day versus 104.4±31.9 mg/day, p=0.243) and divalproex (916.1±212.8 mg/day versus 936.5±336.0 mg/day, p=0.774) during the study period. Lorazepam was received by 50.0% of the patients in the low-dose start group and 68.2% of the patients in the high-dose start group (p=0.164).
Efficacy
By week 6 of treatment, the standard-dose start group showed significant improvements from baseline with respect to the YMRS (-26.5±11.0, p<0.0001), CGI-S (-2.6±1.1, p<0.0001), BPRS (-19.2±9.4, p<0.0001), and HAMD total scores (-5.0±6.0, p=0.001). The YMRS (-16.8±12.1, p<0.0001), CGI-S (-1.7±1.4, p<0.0001), BPRS (-13.8±12.4, p<0.0001), and HAMD scores (-2.4±6.9, p=0.039) were also decreased significantly in the low-dose start group. There were no significant group differences in YMRS (F=2.441, p=0.074), CGI-S (F=2.487, p=0.075), BPRS (F=1.447, p=0.240), and HAMD total score changes (F=1.520, p=0.220) based on repeated measure ANCOVA.
However, as shown Figure 1, the response and remission rates were significantly different between the two groups. The number of patients with a 50% reduction or more in the YMRS total score was 19 (86.4%) at the endpoint in the standard-dose start group and 23 (53.5%) in the low-dose start group (p=0.002). The number of patients entering remission (YMRS ≤12) at the endpoint was 17 (77.3%) in the standard-dose start group and 20 (46.5%) in the low-dose start group (p=0.018).
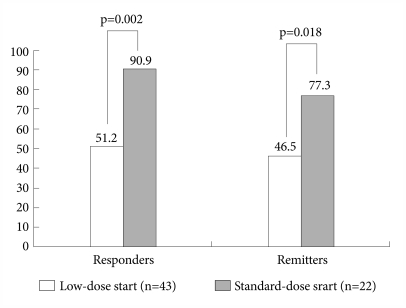
Responder and remission status at study endpoint. Response was defined as a >50% reduction in the Young Mania Rating Scale (YMRS) score from baseline to endpoint. Remission was defined as a YMRS ≤12 at study endpoint. Statistical comparison was performed using chi-square statistic.
Based on logistic regression analyses, being on standard-dose start more than fourfold increased the odds of YMRS response (RR, 4.660; 95% CI, 1.097-19.792; p=0.037) and remission (RR, 4.802; 95% CI, 1.326-17.395; p=0.017) at the study endpoint.
Adverse events and safety
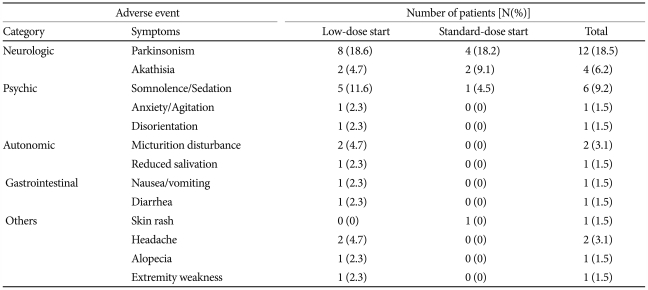
The ziprasidone and divalproex combination was well-tolerated throughout the 6-week study period. Three adverse events led to withdrawal from the study. The reasons for withdrawal were nausea, drowsiness, and headache in one patient each. No statistically significant changes were recorded on the ESRS-behavior rating score, total score for Parkinsonism, total score for dystonia, total score for dyskinesia, CGI scale for Parkinsonism, and CGI scale for dyskinesia at the study endpoint compared to the baseline in both groups. There were no cases of newly emergent tardive dyskinesia. All adverse events were considered mild (n=19, 57.6%) or moderate (n=14, 42.4%). No patient reported serious adverse events.
The adverse events that were reported during the study period were not significantly different between the two groups. Seven patients (31.8%) reported 11 adverse events in the standard-dose start group, and 14 patients (32.6%) reported 22 adverse events in the low-dose start group (χ2=0.004, df=1, p=0.952). Both treatments were generally tolerable, as shown in Table 3. As to extrapyramidal symptoms (EPS), including tremors, rigidity, dystonia, and involuntary muscle contractions, 8 (18.6%) and 4 patients (18.2%) had EPS in the low- and standard-dose start groups, respectively.
The mean weight decreased from 66.3 kg at baseline to 65.6 kg at week 6. The change in weight was not statistically significant (p=0.823). There was no significant group differences in body weight (F=0.461, p=0.501). The change in weight was not correlated with the mean doses for the ziprasidone or divalproex. The weight was increased in 27 patients (41.5%), decreased in 23 patients (35.4%), and unchanged in 15 patients (23.1%); no patient had a statistically significant (≥7%) change in weight.
DISCUSSION
A recent analysis of data from a wide range of fixed- and flexible-dose trials showed that an initial high dose of ziprasidone is associated with better treatment results and a lower risk of subsequent treatment discontinuation in schizophrenic patients than lower doses.7,8 Although these data were obtained from patients with schizophrenia, the patients with bipolar disorder may have similar findings. The results of this study suggest that in a naturalistic setting, initiating a standard dose ziprasidone combined with divalproex was significantly associated with higher response and remission rates than was initiation of low dose ziprasidone combined with divalproex. Moreover, there was no significant difference in tolerability between the low- and standard-dose start groups. Taken together with findings from a similar study in schizophrenic patients,20 these results support the clinical benefits of starting patients at a higher dose of ziprasidone. However, because week 1 ziprasidone dose of standard-dose start group and low-dose start group were significantly different, there is possibility that the rate of dose titration, not only initial dose, can affect the results which favored standard-dose start. Addington et al. also reported that slower titration contributed to a higher dropout rate for ziprasidone during the first two weeks in schizophrenic patients.21
In this study, remission was defined as YMRS≤12.22 However, a cutoff of 8 on YMRS is currently recommended by The International Society for Bipolar Disorders.23 The results of this study should be interpreted with caution in this respect.
It is well-known that the combination of various anti-psychotics and mood stabilizers is more effective than a mood stabilizer alone for patients with acute bipolar mania.24 However, olanzapine,25 risperidone,26,27 and quetiapine28 in combination with a mood stabilizer have been shown to cause significant weight gain. Obesity has been correlated with a poor outcome in patients with bipolar I disorder,29 and other adverse long-term health consequences. Since weight gain during acute treatment may predict a long-term risk for increased body weight and obesity,30 it is important to prevent weight gain during the early treatment of bipolar disorder.
Ziprasidone has been shown to be associated with low risk of significant weight gain.31 Allison et al.32 analyzed 81 studies with respect to weight changes in patients who were treated with anti-psychotics. The weight gain recorded included 0.04 kg for ziprasidone, 2.10 kg for risperidone, 4.15 kg for olanzapine, and 4.45 kg for clozapine, which was estimated by the random effects regression at 10 weeks of therapy. Another comparison study demonstrated that ziprasidone had a more benign weight change than other atypical anti-psychotics, excluding aripiprazole.33 In this study, the ziprasidone and divalproex combination was not associated with weight gain. Because weight gain is greater with other atypical anti-psychotic and mood stabilizer combinations than monotherapy,24 the favorable effect of ziprasidone on weight is an advantage to treating with this medication.
Our study had several limitations. First, because this study did not have a placebo control group, the possibility cannot be excluded that the improvement could simply be due to the natural course of a bipolar disorder. However, the magnitude of improvement in the manic symptoms associated with the treatment argues against this possibility. Second, the washout period was too short; 5 half-lives of many anti-psychotics and mood stabilizers are as long as 7 days.34 Longer washout periods are needed to rule out the effects of previous medications, especially during the early stage of the study. Moreover, the potential confound of previous medication exposure was not addressed. Third, the absence of assessment of other clinical variables, such as a history of past year rapid cycling, an electrocardiogram (ECG), and other laboratory results, was another limitation of our study. Forth, high non-completion rate (32.3%), especially in low-dose start group (17/43, 39.5%) may have affected the results of this study. Because LOCF do not make the unrealistic assumption that subjects who drop out would continue improving as they did at the time of drop out, the response and remission rates at endpoint in group with high non-completion rate could be lower than response and remission rates of group with low non-completion rate. However, the high non-completion rate observed in low-dose start group in this study likely reflects the poor efficacy of low-dose start strategy.
The results of this study should be interpreted with caution in light of the limitations, such as the open design. Moreover, because Asians may respond to lower doses of anti-psychotics due to pharmacokinetic or pharmacodynamic differences,35 the results of this study should be interpreted with some reservations. Indeed, potential interethnic differences in pharmacodynamics and pharmacokinetics may exist which can alter the response to anti-psychotics. Although we conducted careful pre-study education meetings for the investigators, it is possible that observer biases on the rating scales in this multi-center study may have influenced the results. Finally, we did not include a structured diagnostic interview for confirmation of the diagnosis.
In conclusion, despite the many potential methodologic limitations of this open label study, the results of this multi-center, 6-week investigation showed that ziprasidone in combination with divalproex in the treatment of bipolar disorder with acute manic symptoms is safe and effective, especially when ziprasidone is initiated at the standard dose.
Acknowledgments
This study was sponsored and funded by Pfizer, the manufacturer of ziprasidone.