The Correlations among Depressive Symptoms, Cognitive Performance and Serum BDNF Levels in the Patients with Chronic Kidney Disease
Article information
Abstract
Objective
In the current study, we investigated whether there are relations among depressive symptoms, cognitive performance and serum BDNF levels in the patients with chronic kidney disease (CKD).
Methods
Sixty patients with CKD and 65 healthy controls participated. Depressive symptoms were evaluated with Beck depression inventory (BDI) and Hamilton Depression Rating Scale (HDRS). Mini-Mental State Examination included in the Korean version of the Consortium to Establish a Registry for Alzheimer’s disease (MMSE-KC) assessment packet was used for the evaluation of overall cognitive function. To assess memory function, the Korean version of the Hopkins Verbal Learning Test (K-HVLT) was used. BDNF levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit.
Results
The CKD patients showed more depressive symptoms when compared with controls. The depressive symptoms and cognitive function were not associated with serum BDNF levels in the CKD patients.
Conclusion
In the current study, CKD patients had more depressive symptoms when compared controls. However, the serum BDNF levels of CKD patients were not associated with depressive symptoms and cognitive functions. These findings suggested that the serum BDNF levels may not be reflect the cognitive function and depressive mood state in the CKD patients.
INTRODUCTION
Chronic kidney disease (CKD) is a serious medical condition with chronic course and may have dreadful end [1]. Most of patients with CKD spend a large part of their lives receiving treatment, such as chronic ambulatory peritoneal dialysis, hemodialysis, or even kidney transplantation [2]. CKD significantly impairs quality of life and also causes serious problems in mental health, interpersonal relationships, and social functioning [3,4]. Recently, the concerns for mental health of CKD patients has grown and mental health has become one of the important treatment goals in CKD patients [4,5].
It has been reported that major depressive disorder (MDD) is the most common mental illness in CKD patients [3,6]. Although many CKD patients are suffering from depressive symptoms, proper diagnosis and treatment are usually not provided, and this may result in fatal outcomes such as suicide or cessation of CKD treatment [6-8]. There have been studies for searching factors that may be related with MDD [9]. In particular, it has been emphasized the brain-derived neurotrophic factor (BDNF) might play important role in the pathophysiology of MDD [10,11]. BDNF is a neurotrophic factor that is essential for the survival and neuroplasticity of neurons [12,13]. BDNF upregulates various neuroprotective proteins through the activation of mitogen-activated protein kinase (MAPK) signaling pathways [14]. Although BDNF is produced primarily in the central nervous system, it is also secreted from the heart, lungs, kidneys, and spleen [15]. In preclinical studies, it was reported that stress inhibited BDNF-mediated neurogenesis in the hippocampus, and decreased neurogenesis was restored by antidepressant administrations [12,16,17]. In the studies with human subjects, reduced serum BDNF levels were observed in MDD patients [18,19]. The decreased hippocampal and forebrain volumes were observed and these changes in MDD patients are thought to reflect decreased levels of BDNF [20-22]. Additionally, decreased levels of serum BDNF and BDNF mRNA were also observed in the MDD patients, and antidepressant treatment normalized these levels [23,24].
Along with MDD or depressive symptoms, cognitive impairment is commonly observed in CKD patients [25,26]. Uremic toxins in unfiltered blood may induce cognitive impairment and impaired cognitive function also can aggravate MDD or depressive symptoms in CKD patients [27,28]. It has been also known that BDNF is associated with cognitive function [29]. The associations between BDNF Val66Met genetic polymorphism and cognitive function were reported in the studies with human subjects [30,31]. A number of studies revealed that BDNF influences cognitive function by affecting various factors associated with neural plasticity [32-34]. Thus, it is apparent that BDNF plays a role in the pathophysiology of MDD or depressive symptoms and cognitive impairments. Thus, summarizing these findings, it can be postulated that a possible relation among depressive symptoms, cognitive impairment and serum BDNF might be existed. So, we wanted to find out whether there are possible relations among depressive symptoms, cognitive performance and serum BDNF levels in the patients with chronic kidney disease (CKD) in the current study.
METHODS
Study subjects
The subjects in the current study were walk-in patients diagnosed with CKD. A total of 60 patients with CKD participated in study. Selection criteria included 1) between the ages of 20 and 64 years and 2) a diagnosis of CKD lasting more than 3 months. Exclusion criteria included 1) inability to read and comprehend written consent, 2) comorbidity with other medical conditions, 3) currently receiving hemodialysis or peritoneal dialysis, because dialysis affects the levels of serum BDNF, and 4) Prior history of psychiatric illness. To minimize confounding effects, we did not recruit subjects with the comorbidities of psychiatric disorders and medical conditions. The control subjects were healthy individuals who voluntarily visited community health examination center. This study was conducted with the approval of the Institutional Review Boards of Inje University Haeundae Paik Hospital (2011-052) and Busan Paik Hospital (2011-096). All subjects fully understood about study and signed in written consents.
CKD was defined as the glomerular filtration rate (GFR) <60 mL/min/1.73 m2 lasting for 3 months or longer [35,36]. In the current study, CKD is classified into five stages based on the degree of reduction in the GFR: GFR of ≥90 mL/min/1.73 m2 (stage 1, n=2), GFR of 60–89 mL/min/1.73 m2 (stage 2, n=11), GFR of 30–59 mL/min/1.73 m2 (stage 3, n=19), GFR 15–29 mL/min/1.73 m2 (stage 4, n=17), and GFR <15 mL/min/1.73 m2 (stage 5, n=11) [36,37]. We categorized stage 1–3 patients as mild to moderate CKD group and stage 4–5 patients as severe CKD group [36,38]. We also categorized stage 1–2 patients as early-stage group and stage 5 patients as end-stage group [36]. The 60 patients with CKD were categorized according to these five stages based on these classification criteria.
Data assessment
Assessment of depressive symptoms
All subjects were interviewed and rated by psychiatrists. Standardized Korean version of Beck Depression Inventory (BDI) and Hamilton Depression Rating Scale (HDRS) were used for the evaluation of depressive symptoms (8, 9). Cut off scores of normal mood state for BDI is <10 and for HDRS is <7 [39,40].
Evaluation of cognitive function
There have been reported that Mini-Mental State Examination (MMSE) and Hopkins verbal learning test (HVLT) have relatively good sensitivity specificity for the screening of cognitive function and dementia [41-43]. So we adapted MMSE and HVLT for the evaluation of cognitive function. The standardized Mini-Mental State Examination included in the Korean version of the Consortium to Establish a Registry for Alzheimer’s disease (MMSE-KC) assessment packet and the Korean version of the Hopkins Verbal Learning Test (K-HVLT) were also used in the current study [44]. The MMSE-KC, which has scores adjusted for respondents’ age and educational level, consists of 19 items; the maximum score is 30 points. The K-HVLT, which consists of an immediate recall test, a delayed recall test, and a recognition test [45]. In the current study, we did recall test three times and the sum of recall test scores and the delayed recall test score were used as assessment data.
Measurement of serum BDNF levels
To measure serum BDNF levels, venous blood was drawn from the antecubital vein between 09:00 am and 11:00 am. We drew the venous blood in the collection tube without anticoagulant and then separated the serum in an Eppendorf tube using a centrifuge (3,000 rpm, 10 min, 4°C). The serum was stored at -70°C until BDNF analysis. BDNF levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Promega, Madison, WI, USA) [46].
Statistical analysis
The analysis of demographic data and one-way analysis of variance (ANOVA) followed by Sidak’s multiple comparisons test was performed using Graphpad Prism version 7.01 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com. Associations among BDI, HDRS, MMSE-KC, and K-HVLT scores and serum BDNF levels were evaluated using the Spearman correlation test. The level of statistical significance was set at a p-value<0.05.
RESULTS
Demographic characteristics of subjects
Sixty CKD patients and 65 healthy control participated in this study. 25 subjects in the CKD group (29.41%) and 29 in the control group (44.62%) were female gender. The average age of the CKD group was 50.53±10.43 years, and that of the control group was 48.03±9.35 years. We found that there were no statistically significant differences between the two groups in age and gender distributions (Table 1).
Depressive symptoms in the CKD group
The BDI total scores were significantly higher in the CKD group than in the control group (p<0.05), and the HDRS total scores were also significantly higher in the CKD group than in the control group (p<0.05) (Table 1). However, the results of multiple regression analysis with covariates (age and female) revealed that there were no statistically differences in the BDI scores and the HDRS scores. When the CKD patients were divided into groups of those with mild to moderate (stage 1–3) and severe (stage 4–5) CKD, the BDI scores of those in the severe CKD group (p<0.05) were significantly higher than were those in the control group, whereas the HDRS scores of both the mild to moderate and severe CKD groups were significantly higher than were those of the control group (p<0.05). Additionally, the proportion of subjects with BDI scores of ≥10 and subjects with HDRS scores of ≥7 were significantly greater in the CKD group than in the control group (p<0.05) (Table 2).
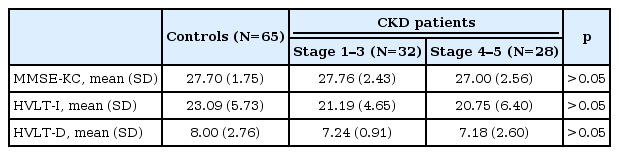
Cognitive function in the CKD group
Overall cognitive function as assessed by MMSE-KC was not significantly different among groups. However, there was statistically difference in the results of multiple analysis with covariates (age and female) (p=0.048) (Table 1). When verbal memory was assessed using the K-HVLT, the CKD group showed significantly lower scores on the recall test (p<0.05) and a tendency toward lower scores on the delayed recall test (p<0.063) compared with the control group (Table 1). The results of multiple regression analysis with covariates (age and female) showed that there were statistically differences in immediate recall test scores and delayed recall test scores between the CKD group and the BDNF group (p<0.05) (Table 1). When the CKD group was divided into the mild to moderate CKD (stage 1–3) and the severe CKD (stage 4–5) groups, there were no significant differences in MMSE-KC scores, immediate recall test scores and delayed recall test scores among the mild to moderate CKD group, the severe CKD group and the control group (Table 3).
Serum BDNF levels in the CKD group
The serum BDNF level was 11.23±1.18 ng/mL in the 65 healthy controls and 11.06±1.07 ng/mL in the 60 patients with CKD and there was no significant difference between the two groups (Table 1). Additionally, the serum BDNF levels were significantly lower in the end-stage (stage 5) group than in the early-stage (stages 1–2) group (p<0.05).
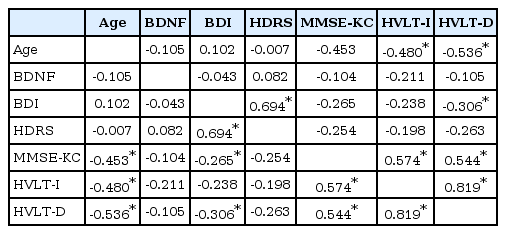
Correlations among depressive symptoms, cognitive functioning, and serum BDNF levels
There were negative correlations between age and MMSE-KC, recall test scores and delayed recall test scores (p<0.05). BDI scores showed statistically significant positive correlations with HDRS scores, MMSE-KC scores and delayed recall test scores (p<0.05). Additionally, HDRS scores also showed statistically significant positive correlations with BDI scores (p<0.05). However, serum BDNF levels in the CKD group were not significantly associated with MMSE-KC scores, K-HVLT recall test scores, BDI and HDRS scores (Table 4). In the current study, it appears that neither depressive symptoms nor cognitive impairments were associated with serum BDNF levels in the CKD group.
DISCUSSION
In the current study, we observed that CKD patients have more depressive symptoms when compared with controls. However, serum BDNF levels were not associated with depressive symptoms or cognitive decline in CKD patients.
MDD is a common mental illness among the CKD patients [3,6]. Although the reported prevalence varies greatly across studies, about 20–30% of CKD patients could be diagnosed as MDD [47]. Comparing with the lifetime prevalence of MDD in the general population, the prevalence among CKD patients is considerably high [3,6,47]. It has been also known that MDD could aggravate the medical condition of CKD by impairing the immune and hormone systems [48]. Thus, early detection and proper treatment of MDD among CKD patients are necessary.
For the detection of MDD, assessment tools such as BDI and HDRS are widely used in clinical settings [49]. BDI is easy to use due to its self-report format, and HDRS has the advantage of adding a clinician’s objective assessment [50,51]. In the current study, BDI and HDRS were used to quantitatively evaluate MDD in CKD patients, and the CKD group was found to have more severe depressive symptoms than the control group. Among CKD patients, the proportion of patients with HDRS scores ≥7 was 35.0%, which was significantly higher than the 15.4% found in the control group. Additionally, the proportion of CKD patients with BDI scores ≥10 was greater that it was in the control group (43.3% vs. 21.5%). These findings are consistent with results of previous studies in which the prevalence of MDD was found to be higher among CKD patients than in the general population [3,4,6].
The current study measured serum BDNF levels in the control group as well as in the CKD group. It has been known that BDNF may play an important role in the pathogenesis of MDD [9,10,31]. The “BDNF hypothesis” of MDD is based on the fact that stress reduce BDNF expression in the hippocampus and that antidepressants can restore BDNF levels [9,52,53].
In previous studies, decreased the levels of BDNF in the hippocampus and in plasma of the patients with MDD were observed [54]. In the studies with CKD patients, it has been reported that female gender is associated with the lower levels of plasma BDNF [55]. Kielstein et al. [56] also reported inverse relationship of BDNF and BDI in chronic hemodialysis patients. However, there were no difference in the BDNF levels between the CKD group and the control group in the current study. Correlation analysis also revealed that there were no correlations between serum BDNF levels and MMSE-KC scores, recall scores, delayed recall scores, BDI scores and HDRS scores. Only the levels of serum BDNF in CKD patients stage 5 were significantly lower than the those of CKD stage 1–2 (data were not shown). It is difficult to estimate with these results alone whether low serum BDNF levels in end-stage CKD patients may be caused by chronic stress or as a result of a hypothalamic-pituitary axis dysfunction [57]. Summarizing these findings, serum BDNF levels measured in the current study were also not associated with scores on clinical rating scales that quantitatively measure depressive symptoms. That is, we were unable to verify the relations between BDNF and depressive symptoms in the current study. The reason for this failure may be due to the scales used in this study. It has been a controversy regarding whether clinical rating scales are suitable for assessing MDD in CKD patients exists. Because the items of BDI and HDRS scales include physical symptoms that patients with CKD may complain regardless of MDD [49].
CKD patients may have functional impairment in the central nervous system because they cannot eliminate uremic toxins that are generated in the body, which could result in cognitive impairment [58]. Cognitive impairment among CKD patients is commonly observed in clinical settings. A previous study reported abnormal digit symbol substitution scores and modified stroop color word scores in CKD patients [59]. In the current study, overall cognitive function as assessed by the MMSE-KC was not significantly different between the CKD patients and controls. However, the results of multiple regressive analysis with covariates (age and female) revealed that there were statistically differences in MMSE-KC scores, HVLT-I and VHLT-D scores between the CKD and the control group. Additionally, serum BDNF levels did not have a significant association with scores on the depression rating scale or the degree of cognitive functioning among CKD patients. Therefore, it may be inferred that the impairment of cognitive functioning in CKD patients may not be associated with BDNF levels in the current study [60,61].
The limitations of this study are as follows. First, the number of CKD patients was small, making it impossible to create sufficiently large groups representing various degrees of renal function impairment. Because cognitive function and serum BDNF levels can vary with the degree of renal function impairment, studies with enough subjects will be needed. Second, we did not use the structured interview for the screening the mental states of the CKD and normal groups. The current study was a cross sectional design and the depressive scores were presentation of the current mood state of CKD patients. So, the structured interview to screen the mental state of subjects should be necessary. Third, the use of drugs and other multiple factors that might influence the serum BDNF levels and cognitive function in CKD patients were not controlled. Thus, further studies will be necessary to obtain more meaningful results if they are able to control for these factors.
In the current study, CKD patients showed more depressive symptoms when compared with controls. The depressive symptoms and cognitive functioning were not associated with serum BDNF levels among CKD patients. In other words, we could not verify BDNF involvement in the development of depression and cognitive impairment.