1. Nayani TH, David AS. The auditory hallucination: a phenomenological survey. Psychol Med 1996;26:177–189.


2. Honda T, Suzuki H, Iwai K, Fujiwara Y, Kawahara H, Kuroda S. Autochthonous experience, heightened awareness, and perception distortion in patients with schizophrenia: a symptomatological study. Psychiatry Clin Neurosci 2004;58:473–479.


3. Amador XF, Strauss DH, Yale SA, Flaum MM, Endicott J, Gorman JM. Assessment of insight in psychosis. Am J Psychiatry 1993;150:873–879.


7. Lera G, Herrero N, González J, Aguilar E, Sanjuán J, Leal C. Insight among psychotic patients with auditory hallucinations. J Clin Psychol 2011;67:701–708.


9. Toh WL, Moseley P, Fernyhough C. Hearing voices as a feature of typical and psychopathological experience. Nat Rev Psychol 2022;1:72–86.

10. West ML. Clinical insights: addressing command hallucinations. Schizophr Res 2018;197:63–64.


11. Norman RM, Malla AK. A prospective study of daily stressors and symptomatology in schizophrenic patients. Soc Psychiatry Psychiatr Epidemiol 1994;29:244–249.


15. Tsang A, Bucci S, Branitsky A, Kaptan S, Rafiq S, Wong S, et al. The relationship between appraisals of voices (auditory verbal hallucinations) and distress in voice-hearers with schizophrenia-spectrum diagnoses: a meta-analytic review. Schizophr Res 2021;230:38–47.


16. Beck AT, Baruch E, Balter JM, Steer RA, Warman DM. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res 2004;68:319–329.


17. Warman DM, Lysaker PH, Martin JM. Cognitive insight and psychotic disorder: the impact of active delusions. Schizophr Res 2007;90:325–333.


18. Palmer EC, Gilleen J, David AS. The relationship between cognitive insight and depression in psychosis and schizophrenia: a review and meta-analysis. Schizophr Res 2015;166:261–268.


20. Ouzir M, Azorin JM, Adida M, Boussaoud D, Battas O. Insight in schizophrenia: from conceptualization to neuroscience. Psychiatry Clin Neurosci 2012;66:167–179.


21. Van Lieshout RJ, Goldberg JO. Quantifying self-reports of auditory verbal hallucinations in persons with psychosis. Can J Behav Sci 2007;39:73–77.

22. Kim SH, Jung HY, Hwang SS, Chang JS, Kim Y, Ahn YM, et al. The usefulness of a self-report questionnaire measuring auditory verbal hallucinations. Prog Neuropsychopharmacol Biol Psychiatry 2010;34:968–973.


23. Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychol Med 1999;29:879–889.


24. Jung SM, Kim MK, Lee JB, Choi JH, Jung BJ, Byun WT. [Reliability and validity of the Korean version of the psychotic symptom rating scale]. J Korean Neuropsychiatr Assoc 2007;46:201–213. Korean.
25. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261–276.


26. Shafer A, Dazzi F. Meta-analysis of the positive and negative syndrome scale (PANSS) factor structure. J Psychiatr Res 2019;115:113–120.


27. Yi JS, Ahn YM, Shin HK, An SK, Joo YH, Kim SH. [Reliability and validity of the Korean version of the positive and negative syndrome scale]. J Korean Neuropsychiatr Assoc 2001;40:1090–1105. Korean.
28. Hwang SS, Chang JS, Lee KY, Kim SH, Ahn YM, Kim YS. Causal model of insight and psychopathology based on the PANSS factors: 1-year cross-sectional and longitudinal revalidation. Int Clin Psychopharmacol 2009;24:189–198.


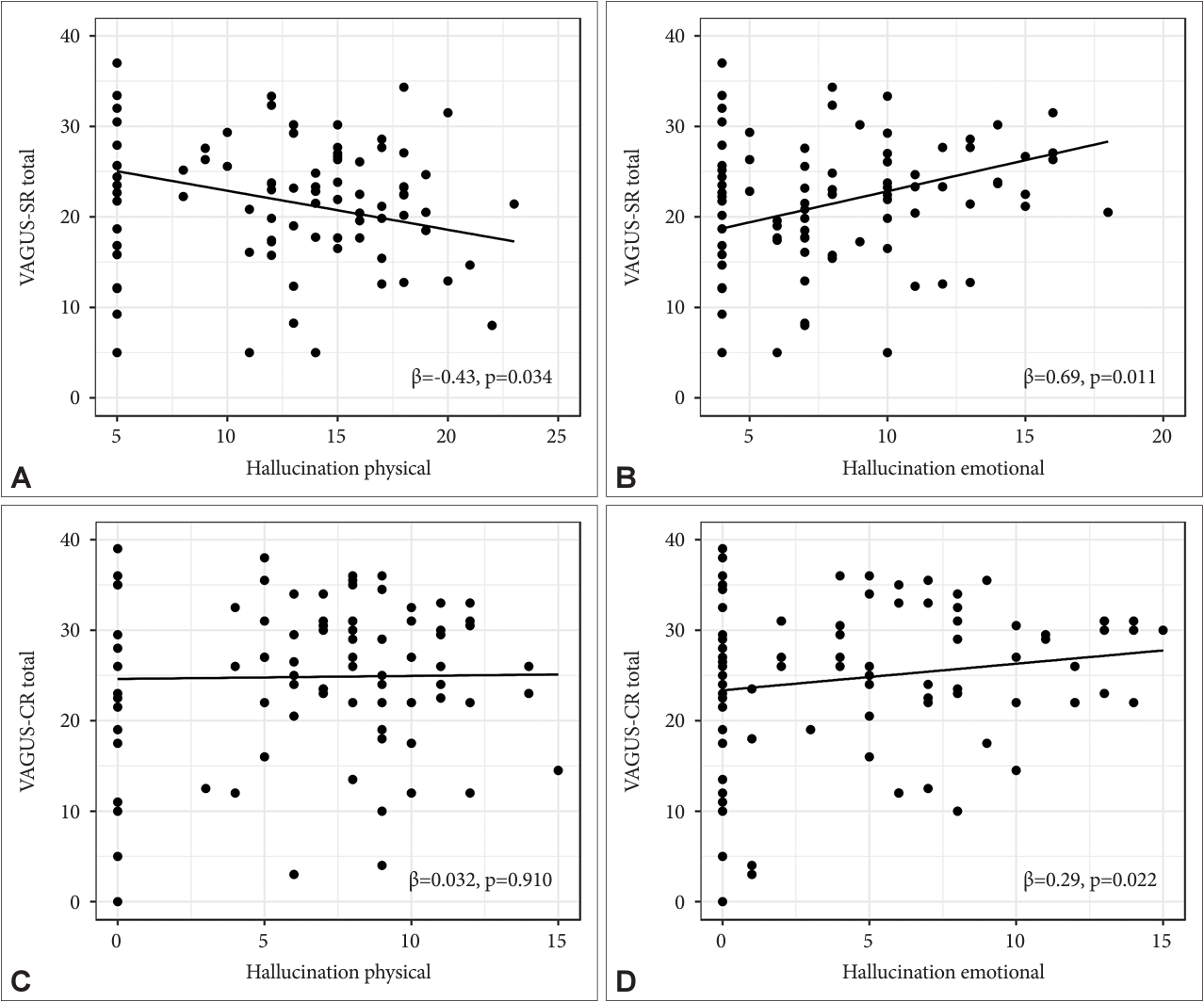
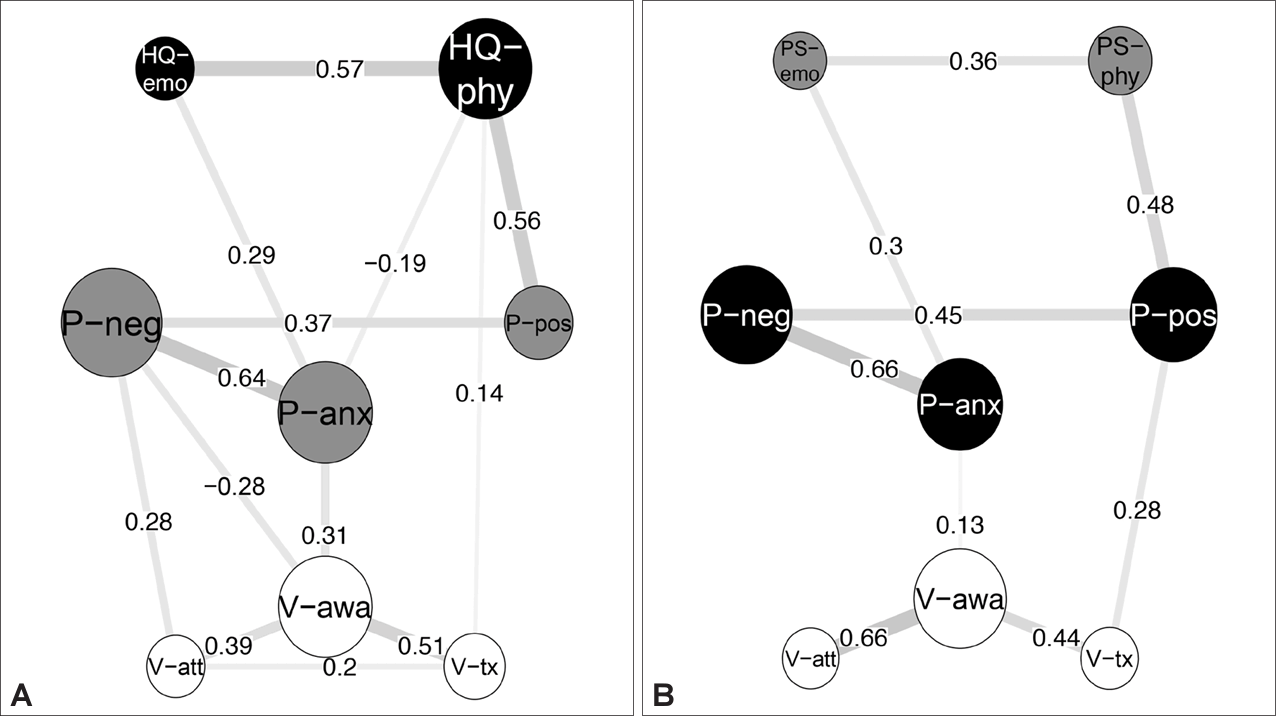
30. Jeong SH, Chung IW, Jung HY, Hwang SS, Kim SH, Youn T, et al. Comparison of clinician-rated and self-report insight in Korean patients with schizophrenia using VAGUS insight scale. Psychiatry Res 2017;258:93–100.


31. Epskamp S, Waldorp LJ, Mõttus R, Borsboom D. The Gaussian graphical model in cross-sectional and time-series data. Multivariate Behav Res 2018;53:453–480.


32. Epskamp S, Fried EI. A tutorial on regularized partial correlation networks. Psychol Methods 2018;23:617–634.


33. Williams DR. Bayesian estimation for Gaussian graphical models: structure learning, predictability, and network comparisons. Multivariate Behav Res 2021;56:336–352.


34. Huth KB, de Ron J, Goudriaan AE, Luigjes J, Mohammadi R, van Holst RJ, et al. Bayesian analysis of cross-sectional networks: a tutorial in R and JASP. Adv Meth Pract Psychol Sci 2023;6:25152459231193334
36. Jöreskog KG. Simultaneous factor analysis in several populations. Psychometrika 1971;36:409–426.

37. Yuan KH, Chan W. Measurement invariance via multigroup SEM: issues and solutions with chi-square-difference tests. Psychol Methods 2016;21:405–426.


38. R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2021.
39. Huth K, Keetelaar S, Sekulovski N, van den Bergh D, Marsman M. Simplifying Bayesian analysis of graphical models for the social sciences with easybgm: a user-friendly R-package. PsyArxiv [Preprint] 2023;[cited November 14, 2023]. Available at:
https://doi.org/10.31234/osf.io/8f72p.

40. van Borkulo CD, van Bork R, Boschloo L, Kossakowski JJ, Tio P, Schoevers RA, et al. Comparing network structures on three aspects: a permutation test. Psychol Methods 2023;28:1273–1285.


41. Rosseel Y. lavaan: an R package for structural equation modeling. J Stat Softw 2012;48:1–36.
42. Birchwood M, Iqbal Z, Upthegrove R. Psychological pathways to depression in schizophrenia: studies in acute psychosis, post psychotic depression and auditory hallucinations. Eur Arch Psychiatry Clin Neurosci 2005;255:202–212.


44. Guerrero-Jiménez M, Carrillo de Albornoz Calahorro CM, GirelaSerrano B, Bodoano Sánchez I, Gutiérrez-Rojas L. Post-psychotic depression: an updated review of the term and clinical implications. Psychopathology 2022;55:82–92.


45. David AS. Insight and psychosis. Br J Psychiatry 1990;156:798–808.


46. Bateson M, Brilot B, Nettle D. Anxiety: an evolutionary approach. Can J Psychiatry 2011;56:707–715.


47. Mayer RE. The search for insight: grappling with Gestalt psychology’s unanswered questions. In: Sternberg RJ, Davidson JE, editor. The nature of insight. Cambridge: MIT Press, 1995, p. 3–32.
48. Holmberg MJ, Andersen LW. Collider bias. JAMA 2022;327:1282–1283.


49. Amador XF, Gorman JM. Psychopathologic domains and insight in schizophrenia. Psychiatr Clin North Am 1998;21:27–42.


51. Mintz AR, Dobson KS, Romney DM. Insight in schizophrenia: a meta-analysis. Schizophr Res 2003;61:75–88.


52. Cavelti M, Kvrgic S, Beck EM, Rüsch N, Vauth R. Self-stigma and its relationship with insight, demoralization, and clinical outcome among people with schizophrenia spectrum disorders. Compr Psychiatry 2012;53:468–479.


57. Gilbert P, Miles JN. Sensitivity to social put-down: it’s relationship to perceptions of social rank, shame, social anxiety, depression, anger and self-other blame. Pers Individ Dif 2000;29:757–774.

58. Cuesta MJ, Peralta V, Zarzuela A. Psychopathological dimensions and lack of insight in schizophrenia. Psychol Rep 1998;83(3 Pt 1):895–898.

59. Hausmann A, Fleischhacker WW. Differential diagnosis of depressed mood in patients with schizophrenia: a diagnostic algorithm based on a review. Acta Psychiatr Scand 2002;106:83–96.


60. Kyzar EJ, Denfield GH. Taking subjectivity seriously: towards a unification of phenomenology, psychiatry, and neuroscience. Mol Psychiatry 2023;28:10–16.

63. Lipsky AM, Greenland S. Causal directed acyclic graphs. JAMA 2022;327:1083–1084.