Comparative Safety of Long-Acting Injectable Antipsychotics: A Systematic Review and Network Meta-Analysis
Article information
Abstract
Objective
To find the safety of long-acting injectable antipsychotics (LAIs) compared to each other, and/or placebo in the treatment of schizophrenia (SCZ) and/or schizoaffective disorder (SZA).
Methods
We performed a systematic review and a network meta-analysis of randomized controlled trials (RCTs) comparing the safety of LAIs versus other LAIs or placebo in adults diagnosed with SCZ or SZA. The primary outcomes were treatment emergent adverse events (TEAEs), serious treatment emergent adverse events (STEAEs), and deaths. The secondary outcomes included treatment discontinuations due to adverse events and all-cause discontinuations.
Results
Seventeen RCTs were included (n=7,908). There were no significant differences between LAIs and placebo in the risk of presenting TEAEs. LAIs had a significant lower risk of presenting STEAEs except for aripiprazole. No significant differences in deaths were found. LAIs showed a significant protective effect against all-cause discontinuation, except for haloperidol. Only aripiprazole had a significantly lower risk of treatment discontinuation due to adverse events.
Conclusion
We found no significant differences in the risk of presenting TEAEs between LAIs and placebo. The majority of LAIs had a significantly lower risk of presenting STEAEs than placebo. Development of international guidelines for the report of safety outcomes related to antipsychotics especially for LAIs in clinical trials could minimize report and interpretation biases and improve the accuracy of posterior meta-analysis.
INTRODUCTION
The development of long-acting injectable antipsychotics (LAIs) in the 1960s was an attempt to improve long-term treatment for schizophrenia (SCZ) and potentially other disorders that could benefit from them [1]. Guidelines and recommendations for the use of LAIs in SCZ have been present for more than 20 years, initially for first-generation LAIs (FGA-LAI) where benefits regarding relapse prevention were remarkably noted [1] and later for second-generation LAIs (SGA-LAI) recommending these for patients who schizophrenic symptoms were under control [2]. Eventually, LAIs recommendations included other chronic mental illnesses and were offered systematically as a first-line pharmacological treatment option for SCZ, schizoaffective disorder (SZA), and delusional disorder [3].
The ultimate aim of LAIs use is to improve treatment outcomes. Evidence shows that this could be reached through increasing adherence, reducing the risk of relapse and hospitalizations, and even lower overall treatment costs in the long term [4]. Longer treatment time with LAIs, lower number of prescribed oral drugs and fewer hospitalizations before LAIs introduction could predict a better global functioning in SCZ or SZA [5]. In a retrospective cohort study with a representative sample of 3,957 users of antipsychotic medication (SCZ, SZA, and others diagnoses), LAIs initiation resulted in lower resource use and overall, lower associated costs when compared with oral antipsychotics (OAPs). Even when LAIs users had higher medication costs, this was offset by lower inpatient and outpatient costs [6]. A 10-year retrospective mirror-image study that evaluated the effectiveness of LAIs compared to OAPs found that hospitalization and emergency visits significantly decreased with the use of LAIs, while planned visits increased in patients treated with LAIs [7]. Unsatisfactory response to OAPs in patients with recent onset SCZ treatment that switch to paliperidone palmitate (PP) was associated with significant improvements in clinical symptomatology and a significant reduction in the number of hospitalizations and days spent in the hospital when compared to retrospective period before initiation of PP treatment [8].
A study that compared treatment costs for patients with SCZ or SZA who were randomized to either risperidone-LAI (RisLAI) or the physician’s choice of an OAP showed a higher mean quarterly outpatient medication cost for the RisLAI group (3,028 USD) compared to the oral medication group (1,913 USD); however total treatment cost did not differ significantly between the two interventions (14,916 USD vs. 13,980 USD; p=0.73) [9]. Similar cost-related benefits were seen in a longitudinal retrospective cohort study in the United States of 32,200 Medicaid users with SCZ that compared treatment patterns, healthcare resource utilization, and spending between those initiated on SGA-LAIs vs. OAPs. Findings showed patients initiated on SGA-LAIs had better adherence and persistence to therapy over 12 months and were associated with lower medical costs despite having considerably higher pharmacy costs relative to OAPs [10].
In a randomized controlled trial (RCT) of 489 participants comparing the effect of LAIs vs. usual care (clinician’s choice including other LAIs) on time to first hospitalization in early phase SCZ; 52 participants (22%) of aripiprazole once monthly (AOM) and 91 of the clinician’s choice (CC) participants (36%) had at least 1 hospitalization, the mean survival time until first hospitalization was 613.7 days for AOM participants and 530.6 days for CC participants with a hazard ratio for time to first hospitalization was 0.56 (95% confidence interval [CI], 0.34–0.92; p=0.02), that favored AOM. Survival probabilities were 0.73 (95% CI, 0.65–0.83) for AOM and 0.58 (95% CI, 0.50–0.67) for CC participants [11]. In other RCT of patients with first episodes of SCZ those who received RisLAI showed lower rates of psychotic exacerbation and/or relapse and better control of breakthrough psychotic symptoms when compared with the OAP (5% vs. 33%, relative risk reduction, 84.7%; p<0.001) moreover medication adherence was a variant associated with these outcomes [12].
Despite LAIs being widely available and having clinical benefits for individuals with SCZ, SZA, and bipolar disorders these agents are still underutilized [13]. Negative attitudes toward medication and substance abuse are consistent reasons for nonadherence to antipsychotic medication among people with serious mental illness [14]. Patients and clinicians may perceive LAIs as coercive, stigmatizing, and as a factor that can negatively affect the clinician–patient relationship [4,15,16]. Ways to overcome this and increase LAIs use include working in a good therapeutic relationship, use of SGA-LAIs over FGA-LAIs, and avoidance of excessive doses to minimize adverse effects [4]. A study specifically designed to address physicians’ attitudes and beliefs towards the treatment of SCZ with LAIs identified a positive correlation between physicians willing to accept the usage of LAIs and the positive attitude of colleagues [17].
Current data about adverse events (AEs) differences among LAIs comes to a large extent from indirect comparisons and spontaneously reported AEs [18].
A meta-analysis comparing safety between LAIs and OAPs in patients with SCZ or SZA showed no significant difference in the incidence of at least one AE (RCTs=7, n=2,686, relative risk [RR]=1.026, 95% CI=0.984–1.071, p=0.231), the incidence of serious AEs (RCTs=6, n=1,848, RR=0.907, 95% CI=0.662–1.242, p=0.542), discontinuation due to AEs (RCTs=14, n=3,570, RR=1.163, 95% CI=0.887–1.524, p=0.275), and deaths (excluding suicide and accident) (RCTs=13, n=3,603, RR=0.695, 95% CI=0.110–4.399, p=0.699) [19]. Likewise, in a recent meta-analysis of LAIs vs. OAPs in the maintenance treatment of SCZ, LAIs showed no significant difference when compared to OAPs regarding most AEs [20].
Because of the lack of clear non-inconsistent safety-related evidence of LAIs for SCZ and SZA, we conducted a systematic review and network meta-analysis comparing safety outcomes between different LAIs and/or placebo.
METHODS
Protocol registration and guidelines
The protocol for this study was registered in PROSPERO under CRD42019128700. This network meta-analysis adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (item checklist can be found in Supplementary Material 1 in the online-only Data Supplement) [21].
Eligibility criteria
Studies should be RCTs of LAIs, meeting the following criteria: 1) patients should be aged 18 years or older with a confirmed diagnosis of SCZ or SZA defined by the fourth or fifth editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV, DSM-IV-TR, and DSM-V); 2) safety assessment through the reported frequency of treatment emergent adverse events (TEAEs), serious treatment emergent adverse events (STEAEs), all cause discontinuations, discontinuations due to AEs and deaths (the authors decided to exclude deaths by homicides since according to the U.S. Food and Drug Administration [FDA] reporting of adverse experiences of biological products, homicides should be classified under unexpected adverse experiences but such specifications were not found in the respective trials) [22]; and 3) treatment duration of ≥12 weeks.
Information sources and search strategy
An experienced librarian with input from the lead researcher, designed and conducted the search strategy in MEDLINE, Embase, Web of Science, and Scopus; from each database’s inception through June 17, 2020, with two actualizations the first on September 14, 2021 and the second on May 31, 2022; references from eligible studies and reviews were also screened for eligibility (search strategy can be found in Supplementary Material 2 in the online-only Data Supplement).
Data management and selection process
Three pairs of investigators independently selected the studies, reviewed the main reports and supplementary materials, extracted the relevant information from the included trials, and assessed the risk of bias. Before the screening phase, pilots were conducted until adequate inter-rater reliability, considered as a Fleiss’ kappa >0.70 [18], was obtained. Any discrepancies were resolved by consensus and arbitration by a panel of investigators within the review team.
Data collection process
A web-based extraction form was created and evaluated by all reviewers before data extraction. General information about the included studies (author, year, country, and design) was extracted.
Outcomes
The primary safety outcome was measured by frequency of TEAEs, STEAEs, and deaths reported during the period after receiving LAIs or placebo until the study ended except for homicides or car accidents. Secondary safety outcomes included all cause discontinuations and discontinuations due to AEs. For each outcome of interest, the intervention with the lowest rate of events was considered the best for the treatment ranks.
Data synthesis
Effect sizes for each treatment comparison reported in the included studies were estimated with odds ratios (ORs), afterwards, frequentist network meta-analysis models were run for each outcome of interest with placebo as the main comparison. Pairwise meta-analytical techniques were used for effect size estimations, random and fixed effect models were explored, if the degree of statistical heterogeneity was considerable (i.e., an I2 statistic >50%), or if the Q test for heterogeneity was significant, the random effects results were considered. Network meta-analysis models were ran considering both random and fixed effects, the assumption of transitivity was explored in network graphs with at least one closed loop by estimating the inconsistency of each model using the Q statistic and the netsplit techniques (i.e., comparing the difference between indirect and direct estimates where closed loops were available in the network graph) if a significant degree of inconsistency was determined the results of the random effects model were reported. No assessment of inconsistency was possible for treatment comparisons without direct estimates. Treatment ranking was performed using the P-score technique and was represented in a forest plot with the pooled effect sizes of each treatment estimated with the network meta-analysis.
After data extraction two groups of studies of interest were identified, the first group included studies where patients with SZA were included, and the second group included studies that used LAIs supplemented with OAPs until study termination not only during LAIs initiation phase. The primary analysis includes results considering these groups of studies along with the other ones. Two sensitivity analyses were conducted excluding each group of studies, and changes on the inconsistency and treatment rank assessments are reported for each outcome. The primary analysis was conducted focusing on the general exposure to the medications of interest, however, we were aware that differences in drug formulations and injection intervals could be a source of inconsistency in the results, thus we performed a third sensitivity analysis comparing different drug formulations and injection intervals according to the reported by each author.
For all analyses of heterogeneity and inconsistency, a p<0.10 was considered as indicative of statistical significance, for all other analyses a p-value threshold of <0.05 was considered. Data analysis was performed using the R software (version 4.1.2; R Foundation for Statistical Computing, Vienna, Austria) using RStudio (version 2022.02.3+492; https://dailies.rstudio.com/version/2022.02.3+492/) and the packages “meta”, “netmeta”, and “dmetar”. The book Doing Meta-Analysis with R: A Hands-On Guide was used for technical guidance throughout the analysis [23].
Risk of bias of individual studies and certainty of the evidence
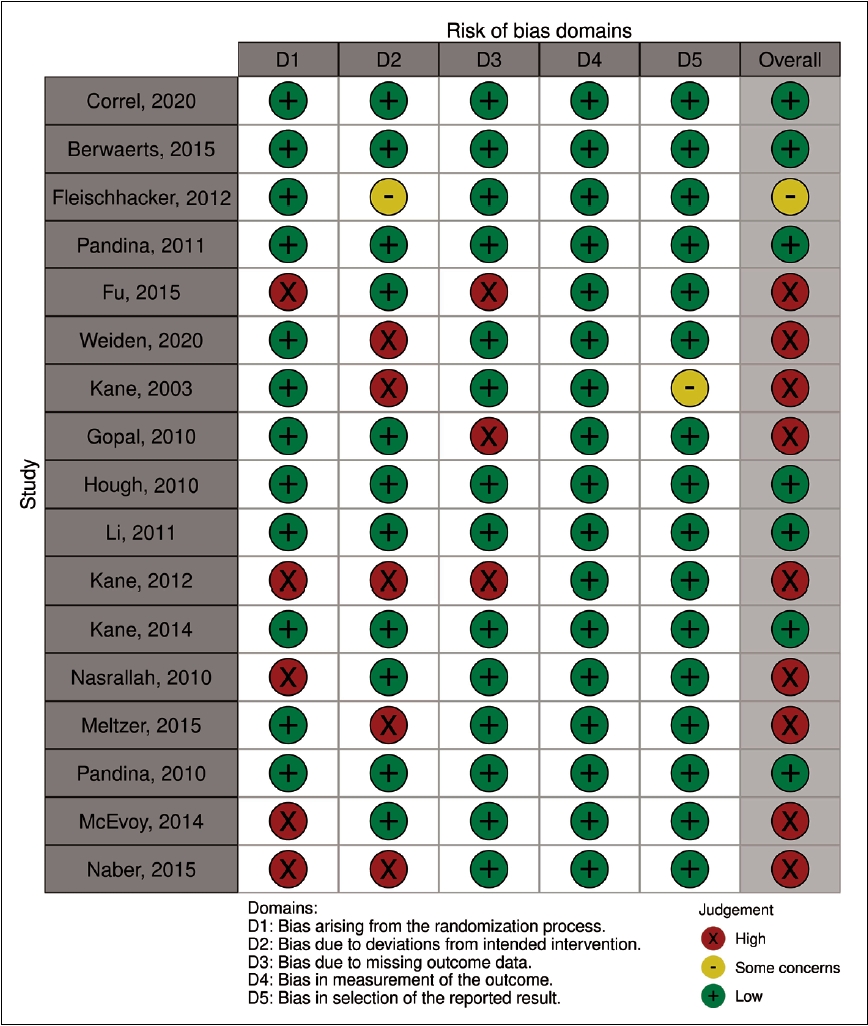
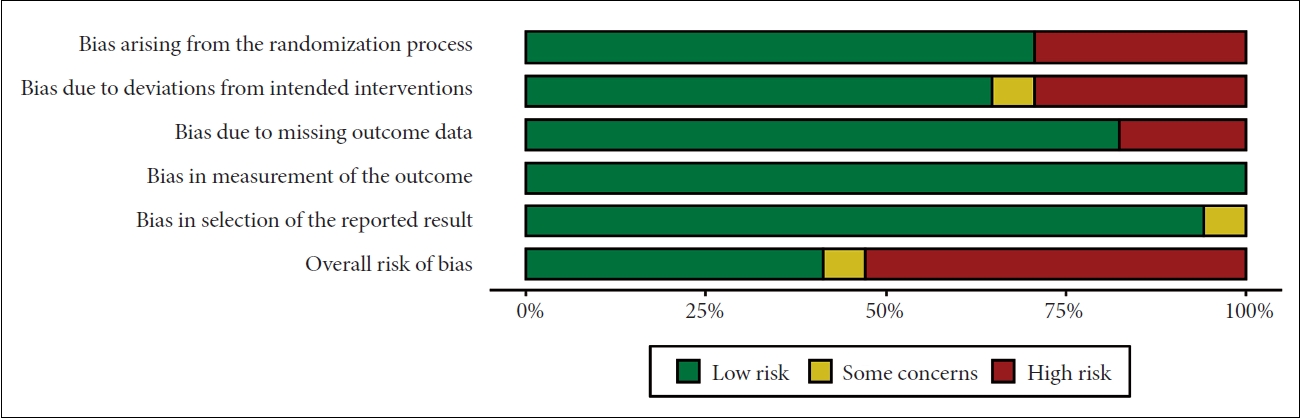
Two reviewers, working independently and in duplicate, evaluated the risk of bias of individual RCTs using the Cochrane risk of bias tool 2.0 (RoB2.0) [24]. This tool evaluates six domains, including bias arising from the randomization process, deviations from the intended intervention, missing outcome data, mismeasurement of the outcomes, and selection of the reported results. According to the tool criteria, the overall risk of bias was classified as low, moderate (labeled “with some concerns’’ by the tool), or high. Any disagreement between the reviewers was solved either by consensus or intervention by a third reviewer. Risk of bias assessment was reported using the robvis data visualization tool [25].
RESULTS
Search
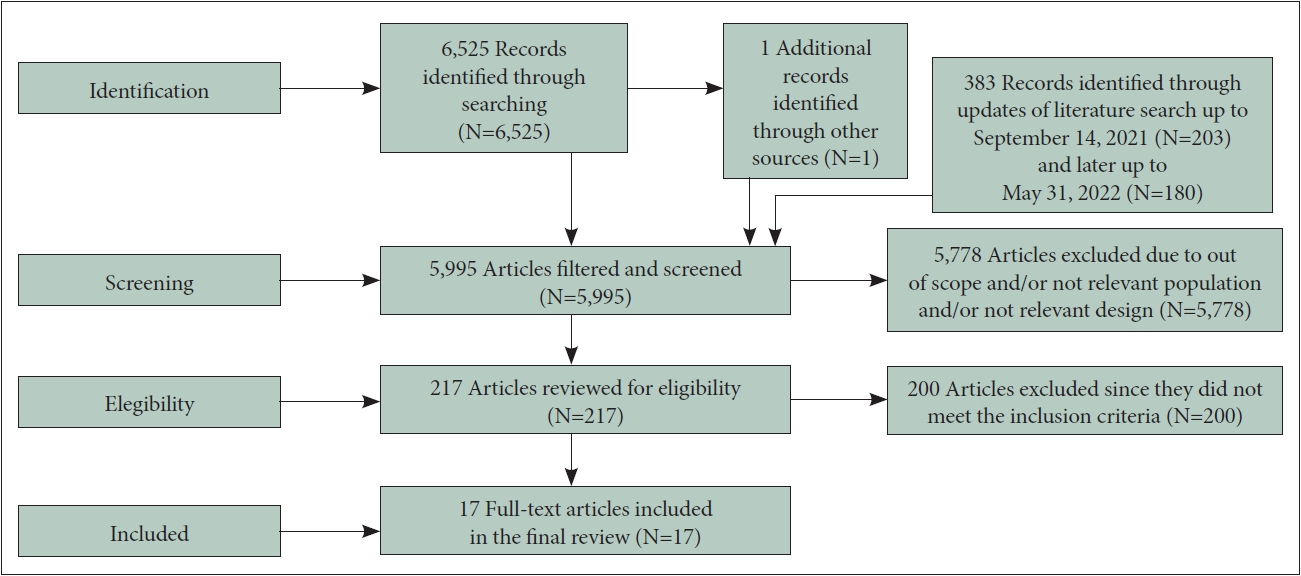
PRISMA flow diagram is presented in Figure 1. We identified 6,525 citations plus 203 from the first and 180 from the second search updates (search results can be found in Supplementary Material 1 in the online-only Data Supplement). Only 17 articles were included in the final review with a total sample of 7,908 patients (safety analysis set population). We included all the extracted data of interest in Table 1.
TEAEs
The effect size estimation for TEAEs resulted in a high degree of statistical heterogeneity (I2=63.7%), thus, the random effects model estimates were used for building the network. Network grafts are presented in Figure 2. Thirteen comparisons were available for the network model, and the resulting graph (Figure 2A) had direct comparisons between all interventions except for the one between aripiprazole-LAI (AriLAI) and RisLAI.

Network grafts. Numbers in each graft are representing the quantity of studies for each comparison. A: TEAEs. B: STEAEs. C: Deaths. D: All-cause discontinuation. E: Discontinuation due to adverse events. TEAEs, treatment emergent adverse events; STEAEs, serious treatment emergent adverse events.
Treatment ranks grafts are presented in Figure 3. Treatment ranking showed that AriLAI had the best-estimated profile across treatments, when compared to placebo (OR=1.01, 95% CI=0.71–1.41), however, none of the estimates were statistically significant (Figure 3A). The primary fixed effects network model had a significant degree of inconsistency (Q=10.2; p=0.006), when considering a random effects model the inconsistency was nonsignificant (Q=2.9; p=0.23). No significant differences between the direct and indirect estimates were detected through the netsplit method (netsplit figures can be found in Supplementary Figure 1 in the online-only Data Supplement).
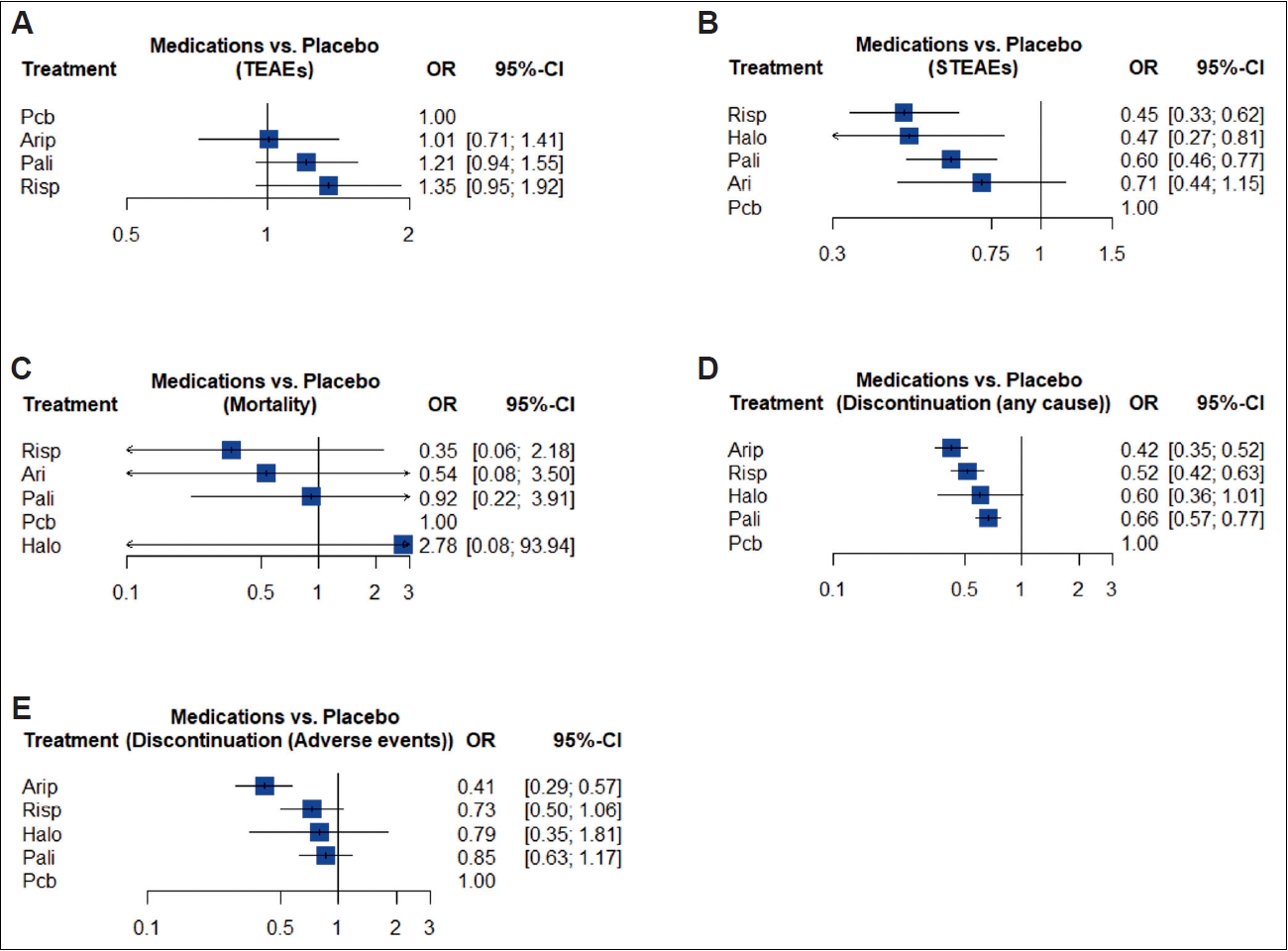
Treatment ranks. Forest plots of relative treatment effects for each active treatment vs. placebo. A: TEAEs. B: STEAEs. C: Deaths. D: All-cause discontinuation. E: Discontinuation due to adverse events. Arip, aripiprazole; Risp, risperidone; Halo, haloperidol; Pali, paliperidone; Pcb, placebo; OR, odds ratio; CI, confidence interval.
Excluding studies that included subjects with SZA had no effect on the heterogeneity for the effect size estimation (I2= 64.4%), the inconsistency of the network model (Q=11.9; p=0.003), or the treatment ranking. Excluding studies combining the use of OAPs and LAIs resulted in a fixed effects network model with a nonsignificant degree of inconsistency (Q=1.8; p=0.18), but with no significant change in the treatment ranking.
Considering the different drug formulations for the analysis the inconsistency of the network model decreased (Q=10.19; p=0.006 vs. Q=6.18; p=0.10), the two formulations of aripiprazole, aripiprazole lauroxil long acting injectable (AriLxLAI) and aripiprazole monohydrate long acting injectable (AriMhLAI) presented considerable differences in their point estimates (OR=0.88, 95% CI=0.65–1.18 for AriLxLAI and OR=1.03, 95% CI=0.78–1.24 for AriMhLAI), however, the only statistically significant estimate was found for risperidone in situ microimplants long acting injectable (RisISMLAI) (OR=3.22, 95% CI=2.04–5.07). Further inclusion of the injection intervals had little effect on the inconsistency of the model (Q=6.03; p=0.05), the administration of AriLxLAI once every month had the best profile (OR=0.84, 95% CI=0.59–1.18) with no statistically significant differences.
STEAEs
The effect size estimation for STEAEs resulted in a high degree of statistical heterogeneity (I2=60.4%), thus, the random effects model estimates were used for building the network. Sixteen comparisons were available for the network model, the resulting graph (Figure 2B) did not have direct comparisons for AriLAI vs. RisLAI and haloperidol-LAI (HDLAI) vs. placebo.
Treatment ranking showed that RisLAI has the best profile across treatments, when compared with placebo (OR=0.45, 95% CI=0.33–0.62), only AriLAI showed a nonsignificant protective effect against STEAEs (Figure 3B). The primary fixed effects model showed a nonsignificant degree of inconsistency (Q=2.27; p=0.32) and was used for the report on STEAEs. No significant differences between the direct and indirect estimates were detected through the netsplit method (Supplementary Figure 2 in the online-only Data Supplement).
Excluding studies which included subjects with SZA had no effect on the heterogeneity for the effect size estimation (I2= 62.3%), the inconsistency of the network model (Q=2.27; p=0.32), or the treatment ranking. However, excluding studies combining the use of OAPs and LAIs lowered the heterogeneity of the effect size estimation (I2=29.3%), had no significant effect on the inconsistency of the network model, but had a significant impact on the treatment ranking with HDLAI showing the best profile across treatments (OR=0.44, 95% CI=0.25–0.77).
Considering drug formulations had no effect on the inconsistency of the network model (Q=2.27; p=0.32 vs. Q=2.35; p=0.50), the only relevant difference in treatment effects was between the risperidone risperdal long acting injectable (RisRsLAI) (OR=0.44, 95% CI=0.32–0.61) and the RisISMLAI formulation (OR=0.70, 95% CI=0.22–2.25), none of the formulations of AriLAI had a significant effect on the outcome. Further inclusion of the injection intervals resulted in a slight decrease of inconsistency in the network model (Q=1.75; p=0.42), the administration of paliperidone every three months was associated with a lower rate of STEAEs compared with its administration every month (OR=0.22, 95% CI=0.07–0.69 vs. OR=0.62, 95% CI=0.47–0.81), similarly, the administration of RisLAI every two weeks resulted in a lower rate of STEAEs that the use of RisLAI every month (OR=0.46, 95% CI=0.33–0.64 vs. OR=0.70, 95% CI=0.22–2.25), however, only one study reported the use of RisLAI monthly formulation.
Deaths
Most studies reported few deaths, with only one study reporting over two deaths and five studies with no reported events on either treatment arm. The effect size estimation resulted in a low degree of statistical heterogeneity (I2=0%), thus, the fixed effects model estimates were used for building the network. Eleven comparisons were available for the network model, the resulting graph (Figure 2C) did not have direct comparisons for HDLAI vs. placebo, HDLAI vs. RisLAI, HDLAI vs. AriLAI, and AriLAI vs. RisLAI.
Treatment ranking showed that RisLAI had the best mortality profile among treatments, when compared with placebo, however, none of the network estimates reached statistical significance (Figure 3C). The primary fixed effects model showed a nonsignificant degree of inconsistency (Q=0.85; p=0.65) and was used for the report on mortality. No significant differences between the direct and indirect estimates were detected through the netsplit method (Supplementary Figure 3 in the online-only Data Supplement).
Excluding studies that included subjects with SZA had no effect on the effect size heterogeneity (I2=0%), the inconsistency of the network model (Q=0.71; p=0.70), or the treatment ranking. Similarly, excluding studies combining the use of OAPs and LAIs had no effect on the effect size heterogeneity (I2=0%), the inconsistency (Q=0.08; p=0.78), or the treatment ranking.
Considering differences in drug formulations and injection intervals showed no differences in the results regarding deaths.
All-cause discontinuation
The effect size estimation for all-cause treatment discontinuations resulted in a high degree of statistical heterogeneity (I2=82.1%), thus, the random effects model estimates were used for building the network. Seventeen comparisons were available for the network model, the resulting graph (Figure 2D) did not have direct comparisons for HDLAI vs. placebo, HDLAI vs. RisLAI, HDLAI vs. AriLAI, and AriLAI vs. RisLAI.
Treatment ranking showed that AriLAI had the best treatment continuation rate among included treatments when compared with placebo (OR=0.42, 95% CI=0.35–0.52). All treatments showed a significant protective effect against all-cause discontinuation, except for HDLAI (Figure 3D). The primary fixed effects model showed a nonsignificant degree of inconsistency (Q=0.58; p=0.75) and was used for the report on all-cause discontinuation. No significant differences between the direct and indirect estimates were detected through the netsplit method (Supplementary Figure 4 in the online-only Data Supplement).
Excluding studies that included subjects with SZA had no effect on the heterogeneity for the effect size estimation (I2= 83.8%), the inconsistency of the network model (Q=0.88; p=0.64), or the treatment ranking. Excluding studies combining the use of OAPs and LAIs decreased the heterogeneity for the effect size estimation (I2=53.5%) and the inconsistency of the network model (Q=0.07; p=0.79), nonetheless, no relevant changes in the treatment ranking were found. Considering drug formulations the inconsistency of the network model increased slightly while remaining nonsignificant (Q=0.58; p=0.75 vs. Q=0.84; p=0.84), AriMhLAI was associated with lower rates of discontinuations compared to AriLxLAI (OR=0.40, 95% CI=0.31–0.52 vs. OR=0.45, 95% CI=0.33–0.60), similarly, RisRsLAI was superior to RisISMLAI (OR=0.48, 95% CI=0.39–0.60 vs. OR=0.65, 95% CI=0.43–0.98). Further inclusion of the different injection intervals had little effect on the inconsistency of the network model (Q=0.21; p=0.89), the administration of AriLxLAI every two months was associated with a lower rate of discontinuation compared to its administration every month (OR=0.37, 95% CI=0.20–0.66 vs. OR=0.48, 95% CI=0.34–0.67) and also when compared to AriMhLAI given every month (OR=0.37, 95% CI=0.20–0.66 vs. OR=0.40, 95% CI=0.31–0.52), similarly, the use of RisLAI given every two weeks was superior to the monthly RisLAI formulation (OR=0.48, 95% CI=0.39–0.61 vs. OR=0.65, 95% CI=0.43–0.98), however, only one study reported the use RisLAI monthly.
Discontinuation due to AEs
The effect size estimation for treatment discontinuation due to AEs resulted in a high degree of statistical heterogeneity (I2=58.7%), thus, the random effects model estimates were used for building the network. Seventeen comparisons were available for the network model, the resulting graph (Figure 2E) did not have direct comparisons for HDLAI vs. placebo, HDLAI vs. RisLAI, HDLAI vs. AriLAI, and AriLAI vs. RisLAI.
Treatment ranking showed that AriLAI had the lowest rate of treatment discontinuation due to AEs (OR=0.41, 95% CI= 0.29–0.57). Only the network estimate for AriLAI reached statistical significance (Figure 3E). The primary fixed effects model showed a nonsignificant degree of inconsistency (Q=2.08; p=0.54) and was used for the report on discontinuation due to AEs. No significant differences between the direct and indirect estimates were detected through the netsplit method (Supplementary Figure 5 in the online-only Data Supplement).
Excluding studies that included patients with SZA had no effect on the heterogeneity for the effect size estimation (I2=53.7%), the inconsistency of the network model (Q=1.86; p=0.40), or the treatment ranking. Excluding studies combining the use of OAPs and LAIs also had no effect on the effect size heterogeneity (I2=53.6%), the inconsistency (Q=1.45; p=0.23), or the treatment ranking.
Considering drug formulations for the analysis slightly increased the inconsistency of the network model while remaining nonsignificant (Q=2.08; p=0.35 vs. Q=5.85; p=0.12), the use of AriLAI remained as the only treatment associated with a statistically significant reduction of discontinuation due to AEs, however, the AriLxLAI was superior to AriMhLAI (OR=0.34, 95% CI=0.21–0.56 vs. OR=0.48, 95% CI=0.31–0.75). Further inclusion of the injection intervals reduced the inconsistency of the network model (Q=0.49; p=0.78), the administration of AriLxLAI monthly was superior to its administration every two months (OR=0.24, 95% CI=0.14–0.43 vs. OR=0.91, 95% CI=0.35–2.40), additionally, the administration AriMhLAI every month proved to be superior to the administration of the AriLxLAI every two months (OR=0.50, 95% CI=0.32–0.79 vs. OR=0.91, 95% CI=0.35–2.40).
DISCUSSION
To our knowledge, this is the first systematic review and network meta-analysis to compare the safety of LAIs in the treatment of adults with SCZ and SZA. Out of the 17 RCT that we reviewed, placebo was used as a comparator in 10, the rest used other LAIs as a comparator. Only one study used a FGA-LAIs, due to this, no special considerations were made in the results for the distinction of FGA-LAIs vs. SGA-LAIs. More so, a previous meta-analysis that compared efficacy and the safety of LAIs vs. placebo in SCZ showed that the risks for all-cause discontinuation, discontinuation due to inadequate efficacy and due to AEs were similar with FGA-LAIs and SGALAIs [43].
The applicability of our results could be considerably limited because we included studies with SZA; however, our sensitivity analysis excluding those articles showed no significant changes for any outcome.
Our findings showed no LAIs had a significant lower risk than placebo of presenting TEAEs; the most frequent TEAEs reported in each study were included in Table 1.
For STEAEs almost all LAIs for the exception of AriLAI had a significant lower risk of STEAEs of which RisLAI had the lowest; nevertheless, HDLAI had the lower risk of presenting a STEAEs once we did not include studies that combine the use of OAPs and LAIs [26-28], all of which used RisLAI plus oral risperidone supplementation.
No significant differences regarding reported deaths between LAIs when compared to placebo. For the exception of HDLAI, all LAIs showed a significantly lower risk of all cause discontinuation whereas AriLAI showed the best continuation rate among LAIs. The risk of discontinuation due to AEs was significantly lower only for AriLAI compared to placebo.
The sensitivity analysis where the use of different drug formulations and injection intervals were considered should be taken with caution due to the few studies or in some cases no more than one trial that include each presentation, however, we had some interesting observations. Regarding drug formulations no statistically significant changes were found in most outcomes except for TEAEs where RisISMLAI had a significant higher risk of presenting a TEAEs than placebo. While the inclusion of the different injection intervals had no statistically significant differences, we found that AriLxLAI administrated every month had the best profile for TEAEs; PP every three months had a better profile than PP every month in contrast to a better profile of RisLAI every two weeks compared to the monthly RisLAI presentation for STEAEs.
We found several concerns related to detection, reporting, and other biases in most of the studies included that should be taken into consideration before the interpretation of our results. Almost all the studies reported events that could be related to the natural history of the disease (psychosis, psychotic disorder, SCZ, SZA, agitation, and hallucinations) as TEAEs or STEAEs which could cause higher frequency in all interventions especially for placebo groups [26-40], furthermore, these studies did not report if those events were related to relapses, exacerbations and/or lack of response. The rest of the studies did not mention these kinds of events nor if those were excluded [41,42].
According to the FDA, regulations for new drugs an “aggregate analysis of specific events observed in a clinical trial (such as known consequences of the underlying disease or condition under investigation or other events that commonly occur in the study population independent of drug therapy) that indicates those events occur more frequently in the drug treatment group than in a concurrent or historical control group.” [22] This kind of analysis could considerably reduce report and interpretation biases, however, no similar considerations were not observed in any of the RCTs included in our study.
One study reported only moderate-to-severe TEAEs, and another study mentioned the most common STEAEs but did not report their totals so we excluded them from their respective outcome comparisons but were included in the rest of the analysis [30,42].
We performed average estimations of TEAEs, STEAEs, and discontinuation due to AEs in studies that reported these outcomes as percentage ranges between groups or subgroups instead of totals [32,33]. One study reported TEAEs and STEAEs separately in three phases (oral conversion, LAI initiation, and LAI continuation) and did not report the total patients with at least one TEAEs or STEAEs since LAI initiation and continuation together; so we decided, in this case, to extract data only from the continuation phase [40]. All reported deaths in the studies except for the exception of homicides or car accidents were included even if they were considered not related to the studied drug by the investigators.
Limitations
Most of the limitations that we encountered were related to the previously discussed reporting biases, however, we considered that their mention represents one of the richest contributions of our study. Also, most of the selected RCTs included patients that were probably severely ill (lack of efficacy, relapse, exacerbation, and inadequate response or adherence) which could have influence significantly in our results, so we encourage further research of clinical trials that compare the safety of LAIs in more stable, less chronic and early diagnosed patients. Another limitation was the use of different drug formulations and injections intervals in each trial and its effects in the inconsistency of our results after performing the correspondent subanalysis for each intervention in every outcome, which if taken into consideration for later investigations could give a richer and more profound insight in how small differences in drug characteristics could impact in safety results and maybe even in the quality of life of the patients.
In conclusion, we found no significant differences in the risk of presenting TEAEs between LAIs and placebo but most LAIs had a significantly lower risk for STEAEs than placebo. Development of international guidelines for the report of safety outcomes related to antipsychotics and specific to LAIs in clinical trials could minimize report and interpretation biases and improve the accuracy of posterior meta-analysis.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.30773/pi.2022.0216.
Netsplit for TEAEs. Arip, aripiprazole; Pali, paliperidone; Pcb, placebo; Risp, risperidone; OR, odds ratio; CI, confidence interval; TEAEs, treatment emergent adverse events.
Netsplit for STEAEs. Arip, aripiprazole; Pali, paliperidone; Pcb, placebo; Risp, risperidone; OR, odds ratio; CI, confidence interval; STEAEs, serious treatment emergent adverse events.
Netsplit for deaths. Arip, aripiprazole; Pali, paliperidone; Pcb, placebo; Risp, risperidone; OR, odds ratio; CI, confidence interval.
Netsplit for all-cause discontinuation. Arip, aripiprazole; Pali, paliperidone; Pcb, placebo; Risp, risperidone; OR, odds ratio; CI, confidence interval.
Netsplit for discontinuation due to adverse events. Arip, aripiprazole; Pali, paliperidone; Pcb, placebo; Risp, risperidone; OR, odds ratio; CI, confidence interval.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Erasmo Saucedo Uribe, Samuel Enrique Olivares Mundo, Raul Ricardo Medrano Garza. Data curation: Samuel Enrique Olivares Mundo, Lorena Martinez Sanchez, Martin Moreno Arellano, Yessica Yaneth Herrera Montemayor, Samantha Berenice Medrano Juarez, Sandra Sabrina Rojo-Garza. Formal analysis: Erasmo Saucedo Uribe, Samuel Enrique Olivares Mundo, Raul Ricardo Medrano Garza, Fernando Diaz Gonzalez-Colmenero, Cesar Bigran Espinosa Cantu, Patricia Lizeth Castillo Morales, Samantha Berenice Medrano Juarez, Sandra Sabrina Rojo-Garza. Investigation: Samuel Enrique Olivares Mundo, Raul Ricardo Medrano Garza, Lorena Martinez Sanchez, Yessica Yaneth Herrera Montemayor. Methodology: Erasmo Saucedo Uribe, Raul Ricardo Medrano Garza, Fernando Diaz Gonzalez-Colmenero, Yessica Yaneth Herrera Montemayor. Software: Fernando Diaz Gonzalez-Colmenero, Cesar Bigran Espinosa Cantu, Martin Moreno Arellano, Patricia Lizeth Castillo Morales, Samantha Berenice Medrano Juarez, Sandra Sabrina Rojo-Garza. Validation: Erasmo Saucedo Uribe, Samuel Enrique Olivares Mundo, Fernando Diaz Gonzalez- Colmenero, Patricia Lizeth Castillo Morales. Writing—original draft: Erasmo Saucedo Uribe, Samuel Enrique Olivares Mundo, Raul Ricardo Medrano Garza, Fernando Diaz Gonzalez-Colmenero, Lorena Martinez Sanchez, Cesar Bigran Espinosa Cantu, Martin Moreno Arellano, Yessica Yaneth Herrera Montemayor, Patricia Lizeth Castillo Morales. Writing—review & editing: Erasmo Saucedo Uribe, Samuel Enrique Olivares Mundo, Raul Ricardo Medrano Garza, Fernando Diaz Gonzalez-Colmenero, Lorena Martinez Sanchez, Cesar Bigran Espinosa Cantu, Samantha Berenice Medrano Juarez, Sandra Sabrina Rojo-Garza.
Funding Statement
None
Acknowledgements
The authors would like to thank César E. Luna Gurrola MD, MPH, for his insightful suggestions and comments for the development of methodology and data analysis.