Collaborative Care to Relieve Psychological Distress in Patients with Medically Inoperable Lung Cancer: Design and Rationale for a Clinical Trial
Article information
Abstract
Psychological distress is common in lung cancer patients with a poor prognosis. The present study aims to investigate the efficacy of collaborative care for patients with newly diagnosed inoperable lung cancer in South Korea. The study is a three-arm parallel-groups nonrandomized clinical trial with an active arm that includes distressed patients who receive collaborative care, one comparison arm that includes distressed patients who receive enhanced usual care, and another comparison arm that includes non-distressed patients. In total, 267 consecutive patients newly diagnosed with medically inoperative lung cancer will be recruited. The primary outcomes are the changes in Hospital Anxiety and Depression Scale-depression and the Distress Thermometer at 12 and 32 weeks after enrollment. Sub-analyses of patients in the active arm of the study will include a comparison of the efficacy of a combination of oral antidepressant (escitalopram) treatment and collaborative care versus that of collaborative care alone.
INTRODUCTION
Psychological distress in cancer patients
Every year, more than 200,000 patients are newly diagnosed with cancer in South Korea [1]. The unmet needs of patients in terms of psychological distress have increased as cancer survival rates have improved [2], and these needs are greatest in the several months following diagnosis [3]. There are several welldocumented risk factors for psychological distress, including younger age, a history of psychiatric illness, poor social support, advanced cancer, functional impairment, and uncontrolled pain [4]. Additionally, biological factors, such as activation of the hypothalamic–pituitary–adrenal axis or low vitamin D levels, are associated with psychological distress [5,6], and genetic components, such as the serotonin transporter (5-HTT) and brainderived neurotrophic factor (BDNF) genes, are responsible for vulnerability to depression in cancer patients [7].
Collaborative care and management of distress
Screening for and management of distress in cancer patients are recommended [4], but very few patients have their psychosocial needs met during cancer treatment [8]. Barriers to the utilization of psycho-oncological services include stigma, preference for self-management, lack of information, need for confidentiality, and a busy schedule [9,10].
Collaborative care models can increase the accessibility of mental health services, and thus may be helpful in overcoming these barriers to successfully managing depression in cancer patients [11]. Several previous randomized trials of collaborative care for cancer patients have been conducted. In the USA, two trials found improvements in depression and QoL in patients with various types of cancer [12,13]. In the UK, the Symptom Management Research Trials (SMaRT) oncology-1 and -2 studies reported similar results for patients with various types of cancer [14,15]. The SMaRT oncology-3 study recruited lung cancer patients found that the collaborative care group exhibited significant improvements in depression, anxiety, and QoL compared to the usual care group [16]. Likewise, a recent metaanalysis of collaborative care interventions reported favorable effects in cancer patients [17].
Studies in lung cancer patients
Advanced lung cancer patients were reported a very poor prognosis; the 5-year survival rate of this population is less than 40% [18]. Consequently, this group of patients has the highest prevalence of psychological distress among cancer patients [19]. More than one-third of lung cancer patients report depression prior to treatment, and this persists in more than 50% of patients [12]. The suicide risk of cancer patients is 12 times higher than that of cancer-free patients, particularly within 12 weeks of the initial diagnosis [20]. Younger age, functional impairment, physical symptom burden, and fatigue are risk factors for distress in lung cancer patients [21,22]. Considering the high prevalence of distress in lung cancer patients, interventions for managing distress are necessary, but few clinical trials have addressed the issue in this population.
Limitations of the previous studies
Despite the promising findings of previous studies, they have had several limitations. Most of these studies were conducted in the USA or the UK [12-17], and a cumulative meta-analysis of collaborative care found heterogeneity among the results of US studies and non-US studies [23]. This heterogeneity could be due to differences in the health care system of each country and/or the degree of fidelity to the original collaborative care model [11]. Thus, trials assessing collaborative care in Asian cancer patients are necessary, but few has been published to date. Meanwhile, of the trials that have been published, few investigated advanced cancer patients, who frequently report severe distress [24], and most enrolled patients were in various phases of cancer treatment even though psychological adaptations to this disease differ by timepoint [25]. Furthermore, no information regarding genetic vulnerability to psychological distress or its relationship with psychological interventions in lung cancer patients is available. Finally, whether the use of collaborative care to manage distress will improve psychological symptoms and/or quality of life to a level similar to non-distressed patients remains unknown.
To overcome the limitations of previous trials, the present authors designed a clinical trial of collaborative care in South Korea. The target population is patients with medically inoperable lung cancer who had a poor prognosis and/or advanced stage of disease.
Trial hypothesis
To ensure homogeneity with respect to the phase of cancer treatment, the enrollment period was limited to patients within 3 months of their initial lung cancer diagnosis. To understand the efficacy of the intervention, the collaborative care intervention group will be compared to two control arms: a group of patients with distress who did not want the intervention and a group of patients without distress. Thus, this study is a non-randomized clinical trial with a three-arm parallel-group design that investigates the efficacy of collaborative care for patients with newly diagnosed inoperable lung cancer in South Korea. The primary hypothesis is that the collaborative care group will report less psychological distress than the enhanced usual care (EUC) group but greater distress than the non-distressed group at 12 and 32 weeks after enrollment. The secondary hypotheses are that QoL, level of functioning, and other psychological/physical symptoms will show greater improvement in the collaborative care group than in the EUC group, but will remain worse than the levels of the non-distressed group. Third, it is hypothesized that a sub-analysis of the collaborative care group will show that psychological distress will improve more rapidly in the patients that receive a combination of oral antidepressant treatment and collaborative care compared to those who receive collaborative care alone.
METHODS
Design
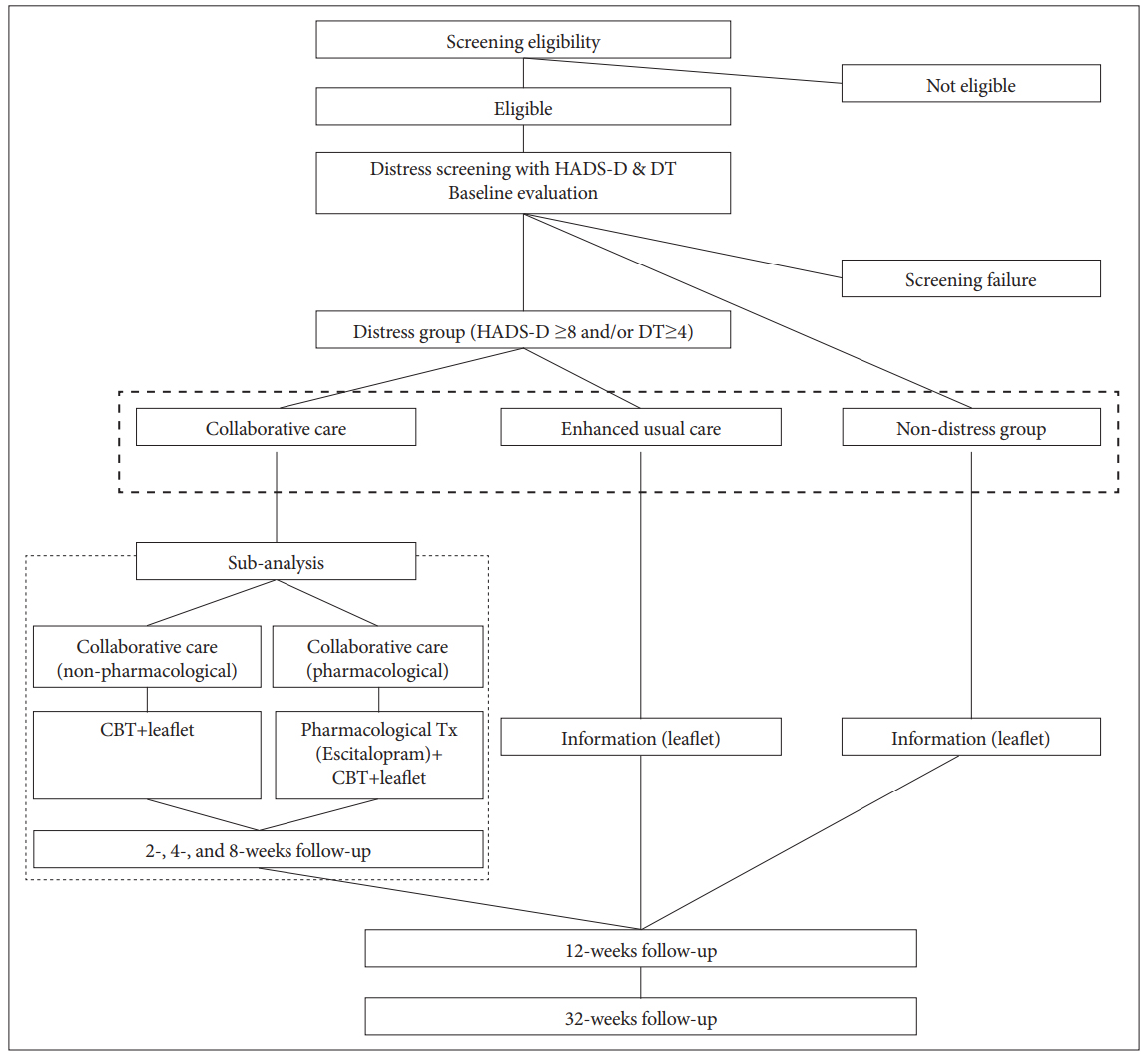
This study is a three-arm parallel-group non-randomized clinical trial that collects primary outcome data at 12 and 32 weeks after enrollment. The CONSORT flow chart of the study is presented in Figure 1.
Recruitment and eligibility criteria
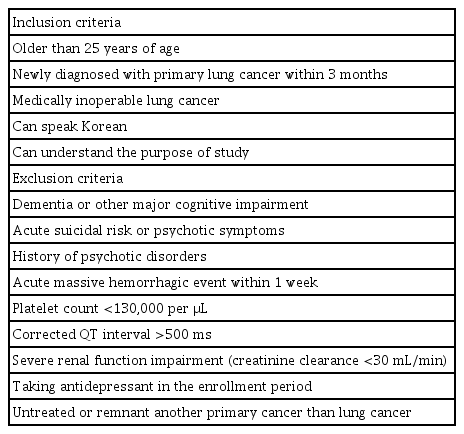
Participants are consecutively recruited from the Lung and Esophageal Cancer Clinic at Chonnam National University Hwasun Hospital, South Korea. The inclusion and exclusion criteria are summarized in Table 1. Because this trial will include a sub-analysis of escitalopram treatment, patients who has an acute hemorrhagic risk, a corrected QT interval >500 ms, and/or a severe renal function impairment are excluded to protect the participants from potential risks [26,27].
Baseline demographic and clinical characteristics
The following sociodemographic and clinical data are obtained via interview or clinical record at baseline: age, sex, years of formal education, history of alcohol and/or smoking, history of depression and/or anxiety, and information about the cancer. Genetic and serological information regarding the 5-HTT and BDNF genes and vitamin D, respectively, are also collected. Personality and religiosity are assessed using the Korean version of the 10-item Big Five Inventory (BFI-10) [28,29] and the Duke religion questionnaire [30,31], respectively.
Participants allocation and treatment
Distress screening and arrangement
Patients who met the eligibility criteria and agreed to participate in the study are screened for distress using the Hospital Anxiety and Depression Scale-depression (HADS-D) [32,33] and Distress Thermometer (DT) [34]. Based on previous studies [33,35], patients who scored ≥8 on the HADS-D and/or ≥4 on the DT at the screening are categorized as distressed, and all others are categorized as non-distressed. Following patient allocation into the distressed and non-distressed groups, patients in the distressed group are categorized into the collaborative care group or the EUC group based on individual preference.
Collaborative care group (Active arm)
The collaborative care model is based on the published literature, i.e., the Depression Care for People with Cancer (DPCP) model [36]. The cancer team (pulmonologists and pulmonology coordinators) and psycho-oncology team (psychiatrist and care manager) care for a lung cancer patient and communicate with each other. The care manager is a trained nurse who delivers the intervention to patients under the regular weekly supervision of a psychiatrist. The intervention consists of eight sessions of low-dose cognitive behavioral therapy (CBT) based on the Oxford Guide to CBT for People with Cancer [37] and Minding the Body: Workbook texts, which have been translated into Korean [38]. These sessions are delivered face to face or via telephone. The first four sessions are delivered to patients every week after group allocation, and the remaining four sessions are delivered to patients every 2 weeks thereafter. Other than Session 1, the order of the sessions can be changed flexibly based on the patient’s physical and/or psychological condition. The patients also receive an educational leaflet three times throughout the 12-week treatment period (Table 2).
It will be also recommended that all patients in the collaborative care group concurrently undergo antidepressant treatment; patients who agreed are prescribed escitalopram (5–20 mg per day) by a psychiatrist. All other interventions in the collaborative care group are the same for patients who agreed and those who do not agree to take medication.
EUC and non-distressed groups (comparison arms)
The EUC and non-distressed groups receive an educational leaflet three times during the 12-week treatment period. These patients also receive the typical clinical management for psychological difficulties, i.e., consultations with and/or visits to the psychiatric department based on their condition and agreement.
Data collection and instruments at the follow-up assessments
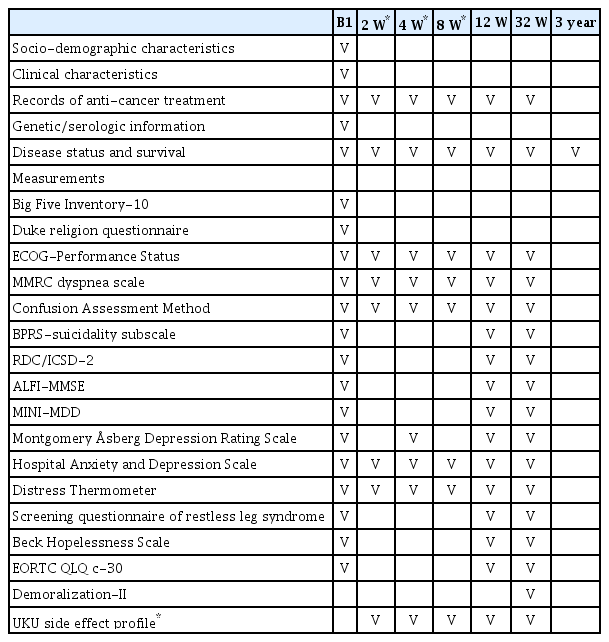
The schedule of assessment for the study is summarized in Table 3. All participants will be followed up at 12 and 32 weeks after enrollment. Additionally, the intervention group will be followed up at 2, 4, and 8 weeks after enrollment to collect detailed data regarding the intervention
Assessment scales
This study included the following assessment scales: Eastern Cooperative Oncology Group Performance Status [39]; modified Medical Research Council (MMRC) dyspnea scale [40]; Confusion Assessment Meth (CAM) [41]; Brief Psychiatric Rating Scale (BPRS)-suicidality subscale [42]; Research Diagnostic Criteria for Insomnia/International Classification of Sleep Disorders-Second Edition (RDC/ICSD-2) [43,44]; Adult Lifestyles and Function Interview of the Mini-Mental State Exam (ALFIMMSE) [45]; DSM-IV criteria for major depressive disorder-the Mini International Neuropsychiatric Interview (MINI-MDD) [46]; Montgomery Åsberg Depression Rating Scale (MADRS) [47]; HADS [32,33]; DT [34]; the diagnostic criteria of the four screening questionnaires of the International Restless Leg Syndrome Study Group (IRLSSG) [48]; Beck Hopelessness Scale (BHS) [49]; European Organization for Research and Treatment of Cancer core questionnaire (EORTC QLQ c-30) [50,51]; and demoralization scale-II (DS-II) [52]. Disease status and survival data will be collected until 3 years after enrollment. Patients who enrolled in the intervention arm also completed the ECOG-PS, MMRC, CAM, HADS, and DT at 2, 4, and 8 weeks after enrollment and the MADRS at 4 weeks after enrollment. Patients who agreed to treatment with escitalopram are also assessed with the Udvalg for Kliniske Undersogelser (UKU) side-effects profile (UKU) side effect profile [53] at 2, 4, 8, and 12 weeks after enrollment.
Primary outcome measures
The primary outcome measures are changes in the HADS-D and DT score at 12 and 32 weeks after enrollment.
Secondary outcome measures
Secondary outcome measures will include changes in the BPRS-suicidality subscale, RDC/ICSD-2, ALFI-MMSE, MINIMDD, MADRS, HADS-anxiety, BHS, EORTC QLQ c-30, DSII, disease status, and survival.
Sample size estimation
The necessary sample size was calculated using the G power tool 3.1 [54] with effect size specification as described by Cohen [55]. Based on a design to produce 80% power to detect a medium effect size (0.25) using Cohen’s f [55] for a repeatedmeasures of analysis of variance (RMANOVA), the assumptions yielded a sample size of 159 patients. Because a previous study reported a 30% rate of cancer-related deaths in advanced lung cancer patients at 32 weeks [16], a dropout rate of 40% was predicted. Thus, a total of 267 patients (i.e., 89 subjects per group) will be enrolled in the study.
Statistical analysis plan
After all outcome data have been collected at 12 and 32 weeks after enrollment, a main analysis of the non-distressed, EUC, and collaborative care arms will be performed using RMANOVA. Additionally, a sub-analysis within the collaborative care arm comparing those who received collaborative care in combination with escitalopram versus those who did not receive escitalopram will be conducted using RMANOVA.
Approval and registration
The trial was approved by the Chonnam National University Hwasun Hospital Institutional Review Board (CNUHH 2014-056) and registered at https://cris.nih.go.kr (registration number: KCT0002127). The sub-analysis of antidepressant treatment in combination with collaborative care was also approved (CNUHH 2014-055) and registered (registration number: KCT0002128).
Acknowledgements
The research was funded by a grant of the Korea Health 21 R&D, Ministry of Health and Welfare, Republic of Korea (HI10C2020) and National Research Foundation of Korea Grant (NRF-2015M3C7A1028899) to Professor JM Kim.
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Jae-Min Kim, Seon-Young Kim, In-Jae Oh, YoungChul Kim. Formal analysis: Sung-Wan Kim, Seon-Young Kim. Funding acquisition: Jae-Min Kim. Investigation: Seon-Young Kim, Cheol-Kyu Park. Methodology: Jae-Min Kim, Il-Seon Shin. Supervision: Il-Seon Shin, Young-Chul Kim. Visualization: Seon-Young Kim. Writing—original draft: Jae-Min Kim, Seon-Young Kim. Writing—review & editing: Jae-Min Kim, Seon-Young Kim, Sung-Wan Kim, Il-Seon Shin, Cheol-Kyu Park, InJae Oh, Young-Chul Kim