The Association between Physical Activity and Anxiety Symptoms for General Adult Populations: An Analysis of the Dose-Response Relationship
Article information
Abstract
Objective
The aim of this study is to determine the dose-response relationship between physical activity and anxiety symptoms.
Methods
We included data of 124,434 participants who had comprehensive health-screening examinations from January 1st, 2012, to December 31st, 2016, in Kangbuk Samsung Hospital, Seoul and Suwon, South Korea. We measured the level of physical activity using the International Physical Activity Questionnaire-short form (IPAQ-SF) and estimated anxiety symptoms using the Beck Anxiety Inventory (BAI). BAI scores of 19 and above were defined as cases. Logistic regression was used to analyze the association between physical activity and BAI-defined anxiety. Furthermore, we assessed whether sex differences might affect the relationship between physical activity and BAI-defined anxiety by stratifying our data.
Results
Compared with the sedentary group (0–600 METs-min/week), individuals achieving 600–6,000 METs-min/wk had a significantly lower risk of BAI-defined anxiety with a U-shaped relationship in general adults. After stratifying our data by sex, we found that optimal ranges of physical activity were 600–9,000 METs-min/wk for men, but 1,200–3,000 METs-min/wk for women.
Conclusion
We identified a U- or J-shaped association between physical activity and anxiety symptoms, suggesting an optimal dose and upper limit of physical activity for decreasing anxiety symptoms. Optimal levels and upper limits of physical activity for reducing anxiety symptoms were higher for men than for women.
INTRODUCTION
Anxiety disorders are the sixth leading cause of global years lived with disability [1]. Anxiety symptoms are associated with negative effects on the individual, including a lower quality of life and comorbid mental illness [2,3]. Furthermore, economic and social burdens related to anxiety disorders are enormous across the developed and developing world [4].
According to various guidelines for treating anxiety disorders, selective serotonin reuptake inhibitors (SSRI) and serotonin-norepinephrine reuptake inhibitors (SNRI) are first-line drugs, and cognitive behavioral therapy (CBT) is regarded as the psychotherapy with the highest level of evidence [5-7]. However, about a third of patients do not respond to antidepressants or CBT [8,9]. Given these criticisms, increasing level of physical activity has been regarded as a new alternative and self-help for relieving anxiety symptoms.
Numerous meta-analyses and systematic review studies have concluded that exercise can significantly decrease anxiety symptoms with a moderate effect size [10-12]. However, studies on the dose-response relationship between physical activity and anxiety symptoms are rare. According to a recent metaanalysis of the association between effect size and level of physical activity using twelve randomized controlled trials, the effect size increased when exercise approached a dose of 12.5 kcal·kg−1·week−1. It then began to decrease when the exercise dose was further increased [13]. Unfortunately, the sample size of that study for the dose-response examination was too small.
Several observational studies have also been conducted to address this issue, and the results are equivocal. Stubbs et al. [14] reported that low physical activity levels are associated with increased prevalence of anxiety by analyzing 237,964 individuals from 47 countries. However, they used a cross-sectional design, categorized levels of physical activity into only two groups, and did not identify sex-specific effect. Brunes et al. [15] found sex differences in the relationship between amounts of physical activity and anxiety symptoms; less than two hours weekly of physical activity with moderate-high intensity was associated with less likelihood of developing anxiety symptoms in healthy men, but not in women. However, only leisuretime physical activity was included as the quantity of exercise, non-leisure time physical activity such as one’s regular occupation, housework, or mode of transportation was disregarded.
Given these aforementioned limitations, our objective was to examine the dose-response relationship between physical activity and anxiety symptoms and find out what amount of physical activity was beneficial for improving anxiety. Additionally, considering sex differences, data were stratified by sex to analyze the effect of sex on the association between physical activity and anxiety symptoms.
METHODS
Participants
A total of 134,639 participants aged 18 to 64 years underwent comprehensive health examinations in Kangbuk Samsung Hospital Health Screening Center between January 1st, 2012, and December 31st, 2016. Those who had a medical illnesses that could affect their level of physical activity were excluded at baseline; such diagnoses included cardiopulmonary disease (angina, myocardial infarction, hypertrophic myocardiopathy, heart failure, heart valve disease, atrial fibrillation, emphysema, chronic bronchitis, and asthma), orthopedic problems (degenerative arthritis and rheumatoid arthritis), and/or neurologic illness (stroke, brain hemorrhage, Alzheimer Dementia, and Parkinson’s disease) [16,17]. In addition, given that psychiatric disorders and psychiatric medications including antidepressants and sleep pills could act as confounding variables, individuals who had psychiatric disorders and took psychiatric medication were also excluded. Since some individuals met more than one exclusion criterion, the total number of patients eligible for this study was 124,434 (Figure 1).
This study’s protocol was approved by the Institutional Review Board of Kangbuk Samsung Hospital (KBSMC 2019-01-042). The requirement for informed consent was waived, because only de-identified data routinely collected during healthscreening visits were used.
Study variables and measurements
Assessment of levels of physical activity
We estimated amounts of physical activity using the Korean version of the International Physical Activity QuestionnaireShort Form (IPAQ-SF) [18]. The reliability and the validity of the Korean version of IPAQ-SF have been evaluated by Oh et al. [19]. Spearman Rho coefficients and Kappa values of test-retest reliability were 0.427–0.646 (median: 0.542) and 0.365–0.620 (median: 0.471), respectively [19]. We used IPAQ-SF to rate the duration at one time, frequency, and intensity of at least 10 minutes of physical activity per session. We calculated duration at each time and frequency as minutes and numbers per week, respectively. Intensity was estimated using metabolic equivalents (MET) and was categorized into three groups: walking, moderate intensity, and vigorous intensity. One MET means the amount of oxygen consumed while sitting at rest (~kcal·min-1 or 3.5 mL O2·kg-1·min-1). We defined moderate intensity of physical activity as 4 METs, meaning activities such as carrying light loads, cycling at a regular pace, or tennis doubles that somewhat increased adults’ breathing rate compared to their normal rate. Vigorous intensity of physical activity was defined as 8 METs, meaning activities such as heavy lifting, digging, aerobics, and fast cycling that significantly increased adults’ breathing rate compared to their normal rate. Walking was defined as 3.3 METs. We calculated amounts of total physical activity, including leisure time physical activity and non-leisure time physical activity, by adding all intensity activities.
Based on the guidelines of both the Centers for Disease Control and Prevention (CDC) and the American College of Sports Medicine (ACSM), 600 METs-min/wk was set as the minimal level of physical activity to have health benefits. We defined individuals who had less than 600 METs-min/wk as sedentary [20].
We established seven categories for MET-min/wk (0 to <600; 600 to <1,200; 1,200 to <1,800; 1,800 to 3,000; 3,000 to <6,000; 6,000 to <9,000; and ≥9,000) by multiples of CDC and ACSM minimal recommended physical activity, ranging from 0–1 times (0 to <600 METs-min/wk) to up to 15 or more times the recommended level (≥9,000 METs-min/wk) [21,22].
Assessment of anxiety symptoms
We measured the severity of anxiety symptoms by using the Beck Anxiety Inventory (BAI). Internal consistency and testretest reliability of the Korean BAI have been reported as 0.91– 0.93 and r=0.84, respectively [23-25]. We defined BAI scores of 19 and above as a clinically anxious state and case [24,26].
Other variables
We identified age, sex, center (Seoul or Suwon), marital status, final degree, employment, income, smoking habit, alcohol intake, medication, and psychiatric history by means of the self-reporting questionnaires of the comprehensive health examination. To estimate alcohol consumption, we used the Alcohol Use Disorders Identification Test (AUDIT) [27]. An InBody 720 machine (Biospace, Seoul, Korea) was used to calculate weight and height to the nearest 0.1 kg and 0.1 cm, respectively. Body mass index (BMI) was calculated as the weight in kilograms divided by height in square meters (kg/m2).
Statistical analysis
Differences in sample characteristics by BAI-defined anxiety were analyzed by a chi-squared test and Student’s t-test for categorical and continuous variables, respectively. Logistic regression was used to calculate odds ratios for BAI-defined anxiety (BAI score ≥19) according to seven physical activity categories with adjustments for age, sex, center (Seoul or Suwon), marital status, final degree, employment, income, alcohol consumption, smoking history, and BMI. In addition, considering correlation anxiety symptoms with depression, we adjusted total scores of depressive symptoms calculated by the Center for Epidemiologic Studies Depression Scale (CESD). We stratified data by sex to analyze the effect of sex differences on the association between physical activity and anxiety symptoms.
Statistical significance was set at a two-sided p<0.05. We did all statistical analyses using Stata version 14.0 (StataCorp LP, College Station, TX, USA).
RESULTS
Table 1 displays group-specific sociodemographic characteristics. Non-anxious subjects were more likely to be married with a higher level of education than anxious subjects. In comparison with non-anxious subjects, anxiety symptoms were more prevalent among those who were females, unemployed, of lower income, and with a higher level of AUDIT and total CESD score.
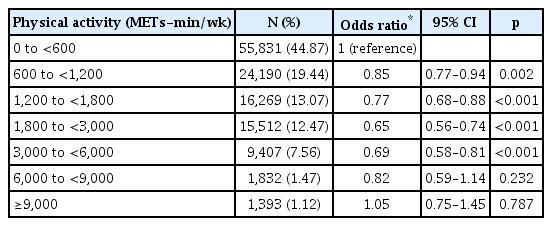
Table 2 shows the results of logistic regression analysis with BAI-defined anxiety as the main outcome variable and the level of physical activity as the principal predictor after adjusting for age, sex, location of the health-screening center, marital status, final degree, employment, income level, alcohol consumption, smoking history, BMI, and CESD score. Compared with the sedentary group (0–600 METs-min/wk), participants achieving 600–6,000 METs-min/wk had a significantly lower risk of case-level anxiety symptoms, with a Ushaped relationship [600–1,200 METs-min/wk: OR, 0.85 (95% CI, 0.77–0.94); 1,200–1,800 METs-min/wk: OR, 0.77 (95% CI, 0.68–0.88); 1,800–3,000 METs-min/wk: OR, 0.65 (95% CI, 0.56–0.74); and 3,000–6,000 METs-min/wk: OR, 0.69 (95% CI, 0.58–0.81)]. We observed that engaging in more than 6,000 METs-min/wk was not associated with the risk of anxiety symptoms. Visual representations of a U-shaped dose-response relationship between total physical activity and the odds ratio of case-level anxiety are shown in Figure 2.

Odds ratio (OR) for total physical activity and anxiety in 124,434 adults. South Korea, 2012–2016. METS: metabolic equivalents, OR: odds ratio.
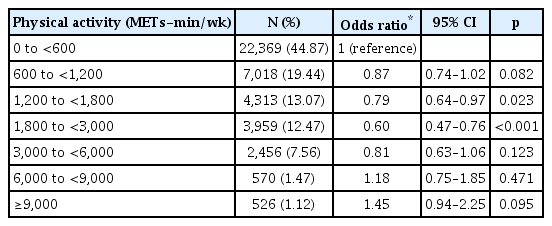
Given sex differences in physical activity and anxiety, we stratified data by sex. Table 3 shows adjusted relationships between physical activity and anxiety symptoms for men, and Table 4 shows these data for women. Compared with the sedentary group (0–600 METs-min/wk), participants performing 600–9,000 METs-min/wk had a significantly lower risk of case-level anxiety symptoms for men [600–1,200 METs-min/ wk: OR, 0.84 (95% CI, 0.73–0.96); 1,200–1,800 METs-min/ wk: OR, 0.78 (95% CI, 0.66–0.91); 1,800–3,000 METs-min/ wk: OR, 0.68 (95% CI, 0.57–0.80); 3,000–6,000 METs-min/ wk: OR, 0.64 (95% CI, 0.52–0.80); 6,000–9,000 METs-min/ wk: OR, 0.61 (95% CI, 0.38–0.98)]. For women, we observed a lower risk of case-level anxiety among individuals performing 1,200–3,000 METs-min/wk [1,200–1,800 METs-min/wk: OR, 0.79 (95% CI, 0.64–0.97); 1,800–3,000 METs-min/wk: OR, 0.60 (95% CI, 0.47–0.76)]. The most beneficial levels of physical activity for lowering the risk of case-level anxiety symptoms were higher for men than for women [men with 6,000–9,000 METs-min/wk: OR, 0.61 (95% CI, 0.38–0.98); women with 1,800–3,000 METs-min/wk: OR, 0.60 (95% CI, 0.47–0.76)] (Figure 2).
DISCUSSION
We found that the relationship between physical activity and anxiety symptoms was curvilinear, suggesting that there were both the most efficacious levels (1,800–3,000 METs-min/week) and upper limits (≥6,000 METs-min/week) of physical activity for decreasing anxiety symptoms. There are potential neurobiological and psychological mechanisms that might explain the anxiolytic effect of physical activity. One mechanism to account for such a benefit is through the action of a brain-derived neurotrophic factor (BDNF). BDNF activates signaling pathways that can result in cognitive improvement and relieve depression and anxiety [28]. Other potential mediations may involve endocannabinoids (N-arachidonoylglycerol and 2-arachidonolylglycerol) [29]. Previous studies have reported that endogenous arachidonate-based lipids can reduce arousal and induce euphoria. Other biological mechanisms for the anxiolytic effects of physical activity might be through regulation of autonomic system functioning [30]. People with anxiety disorders have reduced heart-rate variability (HRV), which can aggravate anxiety symptoms by increased sympathetic and decreased parasympathetic activity. Because exercise regulates autonomic system functioning, anxiety symptoms can be alleviated. There are also psychological theories, including increases in self-esteem and self-efficacy [31]. In addition, exercise can reduce anxiety sensitivity, providing frequent exposure to the anxious situation in the exercise context [32].
For the upper limit of physical activity that provides no significant reduction of anxiety symptoms, numerous hypotheses have been suggested. In terms of biologically detrimental effects of excessive physical activity, the cytokines, hypothalamic, and glycogen hypotheses are most strongly supported. When excessive exercise that can cause tissue microtrauma is prolonged without adequate rest, it may result in chronic inflammation. Furthermore, inflammation is augmented by dysregulation of the stress axis after excessive exercise [33,34]. Pro-inflammatory cytokines, such as TNF-α, IL-1, and IL-6, can increase anxiety symptoms by having negative effects on the brain regions critical for regulation of fear and anxiety (prefrontal cortex, insula, amygdala, and hippocampus) [35]. The other mechanism explaining detrimental effects of excessive exercise is the glycogen-deficit hypothesis. Decreased glycogen causes increased oxidation and decreased levels of branchedchain amino acids (BCAA) known to be involved in central neurotransmitter synthesis as a target for anxiolytics [36]. For the psychologically negative effects of excessive physical activity, lots of studies have suggested that exercise addiction is a behavior addiction, congruous with the work-craving model [37]. The cognitive component of exercise addiction is a negative perfectionism, which is characterized as unrealistic self-expectations, a fear of not trying hard enough, and an implied desire to avoid disapproval that comes from not meeting the expectations of others [38]. Its behavioral component is an underlying obsessive-compulsive drive that make excessive exercise a habitual behavior [39,40]. The hedonic component of exercise addiction is its reduction of negative affect. Because negative affectivity is pervasive when one is unable to exercise, vulnerable people tend to obsess about engaging in vigorous physical activity [37]. These psychological components of exercise addiction, such as negative perfectionism, obsessive-compulsive drive, and negative affectivity, are crucial risk factors for developing anxiety symptoms. To put the risk-to-benefit ratio of exercise into perspective, extreme physical activity could not have positive effects on relieving anxiety.
We found that the most beneficial levels of physical activity for relieving anxiety symptoms were higher for men than for women. According to recent systematic reviews and metaanalyses, intense exercise can result in a significant decrease of both total estradiol and free estradiol, independent of a menopausal state or weight loss [41,42]. Sex hormones contribute to endogenous anxiolytics, such as serotonin and allopregnanolon, thus promoting adaptive responses to stress [43]. Because the reproductive system of women is sensitive to physiological stress such as strenuous exercise, the optimal volume of physical activity might be lower in women than in men.
From the perspective of public health, the recommended level of physical activity for reducing anxiety symptoms can be suggested in real-world terms as follows. In terms of walking at an average pace, 3–45.4 hours per week were efficacious for men and 6–15 hours per week were efficacious for women. In terms of moderate intensity activities, such as carrying light loads, bicycling at a regular pace, and playing tennis doubles, the efficacious amount of physical activity for relieving anxiety symptoms was 2.5–37.5 hours per week for men and 5–12.5 hours per week for women. In terms of vigorous intensity activities, such as heavy lifting, digging, aerobics, and fast bicycling, the appropriate level of physical activity for reducing anxiety symptoms was 1.25–18.75 hours per week in men and 2.5–6.25 hours per week in women.
Our study had several limitations. First, since we measured both exposure and outcome simultaneously, there might be some reverse causation (anxiety leading to a reduced level of exercise). Second, although we attempted to account for several confounding variables, several crucial potential confounders such as diet, personality, and attitude toward health, could not be measured. Third, physical activity and depression were measured by means of self-report screening tools with a risk of reporting bias. Fourth, we defined anxiety as having a BAI score of 19 and above. The operationalization of anxiety symptoms via a self-rating scale cannot be considered equivalent to a clinical diagnosis. Thus, a risk of misclassification remains.
Our study also had several strengths. First, using a large sample, we identified the dose-response relationship between physical activity and anxiety symptoms. Second, considering the correlation between depression and anxiety, we adjusted for depression to clarify the association between anxiety and physical activity. Third, given the effect of sex differences on the relationship between anxiety symptoms and physical activity, we stratified data by sex and identified efficacious sex-specific levels of physical activity for reducing anxiety symptoms.
Acknowledgements
We dedicate this paper to the spirit of the departed, Professor Se-Won Lim.
Notes
The authors have no potential conflicts of interest to disclose.
Authors’ contribution
Conceptualization: Sun-Young Kim. Data curation: Young-Chul Shin, Sun-Young Kim, Mi Yeon Lee. Formal analysis: Sun-Young Kim, Mi Yeon Lee. Funding acquisition: Kang-Seob Oh. Investigation: Sun-Young Kim. Methodology: Sun-Young Kim, Mi Yeon Lee. Project administration: YoungChul Shin, Sang-Won Jeon. Resources: Sun-Young Kim. Software: Sun-Young Kim, Mi Yeon Lee. Supervision: Sang-Won Jeon, Dong-Won Shin, WeonJeong Lim, Young-Chul Shin, Kang-Seob Oh. Validation: Sun-Young Kim. Visualization: Sun-Young Kim. Writing—original draft: Sun-Young Kim. Writing—review & editing: Sun-Young Kim, Kang-Seob Oh.