Chronobiology Revisited in Psychiatric Disorders: From a Translational Perspective
Article information
Abstract
Objective
Several lines of evidence support a relationship between circadian rhythms disruption in the onset, course, and maintenance of mental disorders. Despite the study of circadian phenotypes promising a decent understanding of the pathophysiologic or etiologic mechanisms of psychiatric entities, several questions still need to be addressed. In this review, we aimed to synthesize the literature investigating chronobiologic theories and their associations with psychiatric entities.
Methods
The Medline, Embase, PsycInfo, and Scopus databases were comprehensively and systematically searched and articles published between January 1990 and October 2019 were reviewed. Different combinations of the relevant keywords were polled. We first introduced molecular elements and mechanisms of the circadian system to promote a better understanding of the chronobiologic implications of mental disorders. Then, we comprehensively and systematically reviewed circadian system studies in mood disorders, schizophrenia, and anxiety disorders.
Results
Although subject characteristics and study designs vary across studies, current research has demonstrated that circadian pathologies, including genetic and neurohumoral alterations, represent the neural substrates of the pathophysiology of many psychiatric disorders. Impaired HPA-axis function-related glucocorticoid rhythm and disrupted melatonin homeostasis have been prominently demonstrated in schizophrenia and other psychotic disorders, while alterations of molecular expressions of circadian rhythm genes including CLOCK, PER, and CRY have been reported to be involved in the pathogenesis of mood disorders.
Conclusion
Further translational work is needed to identify the causal relationship between circadian physiology abnormalities and mental disorders and related psychopathology, and to develop sound pharmacologic interventions.
“There is a time for many words, and there is also a time for sleep.” Homer, 850 BC
INTRODUCTION
Rhythmicity is a fundamental characteristic of the nature of life. Time as a dynamic and complex phenomenon, plays a pivotal role to sustain rhythmicity for the biologic essentials and needs of living organisms. Chronobiology aims to define basic principles of vital reactions that occur nearly 24 hours per day through circadian rhythms and biologic processes in anything from single cells to human beings. The first scientific awareness of circadian rhythms started with observations of the mimosa plant (Mimosa pudica) folding independent of daylight by the French astronomer Jean Jacques d’Ortous de Mairan, in 1729 [1]. In the 1930s, the German biologist Erwin Bünning subsequently noticed that the movement of the bean plant had an intrinsic period that did not change under constant light conditions and inferred that such periodic alterations were arranged with an endogenous clock [1].
The term ‘circadian’ was first used by Franz Halberg in 1959. It means ‘about a day’ and an endogenous day slightly shorter or longer than 24 hours (from the Latin term circa: about and diem: day) depending on constant conditions, preserved from environmental factors [2]. Uncovering interactions between molecules and cells within an endogenous day was a major advancement in the discovery of the essential mechanism of circadian rhythm, which was a remarkable scientific milestone in chronobiology. It had been eagerly attempted to explain the further molecular mechanisms of circadian rhythm; however, the oscillation process could not be unraveled until 1971. Konopka and Benzer [3] first determined a gene by observing the differences of circadian period lengths among three mutant flies. They demonstrated three mutants, one was arrhythmic, another had a shorter period of 19 h, and the third had a longer period of 28 h; flies with neither the short-period gene nor the long-period gene or the arrhythmic gene would not produce a normal rhythm. They concluded that the same functional gene with a point mutation appeared to be affected in all cases. This work inspired Jeffery C. Hall, Michael Rosbash, and Michael Young, independently. They cloned and rescued the Drosophila Period gene, which was recognized as the first clock gene, found in 1984 [4,5]. They defined the transcriptional translational feedback loop (TTFL) model with the analysis of Per gene expression and they demonstrated additional genes and proteins in further work. The simple genetic model they postulated revealed the generation of an autonomous oscillator, including transcription-translation cycles from interacting positive and negative feedback loops that depend on ribonucleic acid (RNA) and protein levels, which is still used to understand circadian rhythms. Consequently, they were awarded the Nobel Prize in Physiology and Medicine in 2017 for their explanatory findings of molecular mechanisms controlling the circadian rhythm [6].
Despite the fact that the understanding of the neural basis of rhythmicity and central nervous system (CNS) involvement in circadian mechanisms is not long-standing knowledge, the discovery of the suprachiasmatic nucleus (SCN) of the anterior hypothalamus, which was later described as the master circadian pacemaker in mammals, is actually not very recent. The SCN was first defined as a cluster of different neurons in the 1880s and was subsequently recognized in a number of mammalian species’ brains through comparative studies of the hypothalamus by Crosby and Woodburne [7,8]. However, the discovery of its regulatory function on circadian rhythm occurred nearly 100 years later. The SCN contains a complex neurochemical organization and its functional organization had been revealed with comprehensive experimental studies regarding the function of localization, the neuronal mini-network it contains, and its role in the circadian system. Consequently, the SCN is recognized as a coordinator of biologic processes regulating numerous cellular clocks of the brain and other organ systems.
The findings of considerable studies revealing that a broad range of cell types in the body and brain have biologic clocks raised questions regarding the specific function of circadian rhythm and its contribution to illnesses. Circadian rhyhthms in peripheral organ systems and their impeccable relationship with the SCN and other physiologic and metabolic mechanisms are essential for physical and mental health. The internal desynchronization between the central and peripheral clocks which may be a result of shiftwork or diversity of clock genotypes or circadian rhythm sleep disorders including delayed sleep phase disorder, advanced sleep phase disorder, non-entrained type, irregular sleep-wake rhythm, shift work sleep disorder and jet lag disorder have been associated with many illnesses including metabolic dysfunctions, obesity, cancer, and mental disorders [9,10].
Circadian rhyhtms disruption refers to a range nosological penumbra that includes changes in phase and amplitude of circadian rhythms, circadian misalignment, altered phase relationship between the sleep-wake cycles and endogenous circadian rhythms. Therefore, we would like to provide brief information about descriptions of circadian rhyhtms disruption types. The amplitude of a function is the distance between the mean value and the peak. Therefore, the amplitude resembles half the range of oscillation. Amplitude of the circadian rhythm is involved in the sleep-wake cycle and the changes in amplitude lead to the changes of timing and consolidation of sleep and wakefulness [11]. Phase is also one of the parameters that characterize a circadian rhythm. The circadian pacemaker is known to drive a number of physiologic variables including body temperature and the rhythms of melatonin and cortisol through suprachiasmatic nuclei [12]. The phase of the circadian rhythms is mostly determined by both genetic and environmental factors such as routine daily activities and sunlight. The internal phase advance of biological rhythms which reflects any disturbance of either sleep-wake cycle or endogenous circadian rhythms is related to illnesses and aging [11]. The term “circadian misalignment” describes a range of circumstances, such as inappropriately timed sleep and wake, misalignment of sleep/wake with feeding rhythms, or misaligned central and peripheral clocks [13]. Another subtype of circadian misalignment includes the misalignment of body rhythms with environmental cycles that is usually found in night-shift workers characterizes a condition of chronic desynchronization similar to that produced by persistent jet lag. Different types of circadian misalignment have been associated with increased risk for both physical and psychiatric disorders [13].
Circadian rhythms abnormality, a common manifestation of nearly all psychiatric disorders, is not a surprising predisposing factor for mental disorders, because sleep is considered as a cardinal psychological and vital function and requires routine evaluation in every mental state examination. The common disrupted mechanisms related to the circadian rhythm in psychiatric disorders could be determined as the melatonergic system, its effects of sleep pattern, and the hypothalamus-pituitary-adrenal (HPA) axis. Besides, studies of human circadian rhythm genes revealed that genetic polymorphisms of these genes predisposed to psychiatric disorders [14-16]. Therefore, circadian disturbances seem to be the common thread to all these possible underlying mechanisms that contribute to illness onset, maintenance, and even the response to treatment. Special attention ought to be paid toward the physiology and pathology of circadian rhythm to understand the etiology of psychiatric disorders, and to develop appropriate treatment strategies because chronobiology is an essential field of work in mental disorders. Related literature provides information on circadian rhythm disturbances for certain psychiatric diagnoses such as schizophrenia, mood and anxiety disorders. However, we are aware of a lack of a comprehensive perspective of molecular and neural substrates of common disrupted circadian mechanisms to clinical manifestations in psychiatric disorders. There have been recent reviews relevant to the subject. For instance, Jones and Benca [17] reviewed circadian disruptions in psychiatric disorders, however, they only focused on schizophrenia and mood disorders. Wulff et al. [18] and colleagues comprehensively reviewed sleep and circadian rhythms disruption in psychiatric disorders. Nevertheless, their review was lack of findings regarding current genetic, molecular and neurohumoral models of circadian pathologies. Therefore, we aimed to present a comprehensive review from a translational perspective regarding the reciprocal relationship between neurobiologic underpinnings of circadian rhythm pathologies and psychiatric disorders in this article.
Searching strategy and selection criteria of reviewed studies
An electronic database search was performed by the authors in the MEDLINE, Embase, PsycInfo, and Scopus databases for relevant articles published between January 1990 and October 2019. We searched reference lists of relevant reviews. Different combinations of the keywords psychiatric disorder, mental disorder, mood disorder, bipolar disorder, depression, unipolar depression, major depressive disorder, schizophrenia, psychotic disorders, anxiety disorders, circadian rhythms, circadian markers, chronotype, chronobiology, circadian gene, clock gene, melatonin, and HPA axis were polled. Articles published only in English were reviewed. Unpublished studies, case reports, theses, and conference papers were excluded. Several highly cited and regarded comprehensive review articles and meta-analyses are cited due to space considerations. Eligible open-access and institutional-access articles were recruited. The articles were filtered through an inspection of the abstracts in order to select the most suitable articles related to the topic. In addition to database searches, the reference lists of the relevant articles were also evaluated manually for additional publications matching the scope of our review. The authors avoided incorporating duplicated samples of the key papers; however, studies with similar methodology were included if they provide essential findings to the literature. The visulised algorithm of the article selection is shown in Figure 1.
MOLECULAR REGULATION OF THE CIRCADIAN RHYTHM
We believe that it is noteworthy to briefly summarize the molecular underpinnings of circadian science that gave input to the research into neural substrates of rhythmicity. Although the aforementioned discovery of the period gene was a remarkable finding that identified a genetic determination of the biological clock, it did not mean comprehension of all circadian molecular mechanisms. The circadian rhythm started to be more understandable with the determination of alterations in PER protein and period mRNA levels during a day. Hardin et al. [19] ascertained that levels of period mRNA peaked in the early night, several hours earlier than the peak PER protein abundance. The TTFL model emerged with the discovery of further circadian rhythm genes found in subsequent studies. According to this model, PER and TIM (a protein encoded by the timeless gene) proteins transformed into a heterodimer form in the cytoplasm in order to translocate into the nucleus. TIM protein allows nuclear entry of PER [20]. Besides CLOCK and CYCLE {orthologues of mammalian CLOCK and BMAL-1 [a protein encoded by the brain muscle ARNT-like protein-1 (Bmal-1) gene], respectively} constitute a protein couple that supports the transcription of period and timeless genes [the equivalent of period 1–3 and cryptochrome 1–2 (Cry) in mammalian cells] in the nucleus [21,22]. When the PER-TIM heterodimer binds to the CLOCK-CYCLE couple, CLOCK-CYCLE segregates from DNA and the transcription of downstream genes related to PER and TIM conclude. In other words, the PER and TIM heterodimer terminate their transcription. However, in the event of a decrement in PER and TIM protein levels, the CLOCK and CYCLE couple activates their transcription once again, and TTFL starts over. All of these biochemical reactions include transcription and translation processes that occur rapidly. However, a near 24-h period needs a delay period and timeless gene transcriptions. The explanation about the regulation of the needed delay comes from the discovery of the doubletime gene, another member of the clock genes [23,24]. The doubletime gene’s product casein kinase-1 (CSNK-Iε; casein kinase 1 epsilon in mammals) phosphorylates PER for degradation. Thus, activity of the doubletime gene reduces the stability and accumulation of PER, thereby promoting a delay between PER-TIM transcription and PER-TIM nuclear function [6,25]. This molecular mechanism occurs both in the SCN and nearly all peripheral cells.
The maestro of chronophysiologic rhythms including body temperature, sleep-wake cycle motor activity, and neuroendocrine functions, is located in the SCN of the hypothalamus. The clock genes in the peripheral cells such as hepatocytes, adipocytes or epidermal and dermal cells have their own rhythmicity; however, cyclic processes in which the SCN is involved provide an integrative organization of the physiologic functions and behavioral outputs of the body [26,27]. The circadian system sustains an endogenous rhythmic activity in spite of environmental cues. Regardless of the presence of light, the neuronal activity in the SCN occurs at a higher frequency during the day compared with the night. The neurons of the SCN tend to be excitable in the day to maintain spontaneous activity through persistent Na++ currents, oscillations in chloride pumps, K+ channels, and Ca++ pools in the morning. Conversely, hyperpolarized neurons are inhibited and keep the silence in the SCN at night [28]. CRY and PER proteins gather in the cytoplasm before translocating into the nucleus where they inhibit CLOCK-BMAL-1 activity during the night. In other words, CRY and PER proteins terminate their own transcription when they inhibit CLOCK-BMAL-1 complex activity. After that, degradation of PER and CRY manages the inhibition of CLOCK-BMAL1 toward the morning, followed by resumed transcription of period/cryptochrome and other clock genes [29].
Involvement of master clock in the regulation of circadian rhythm
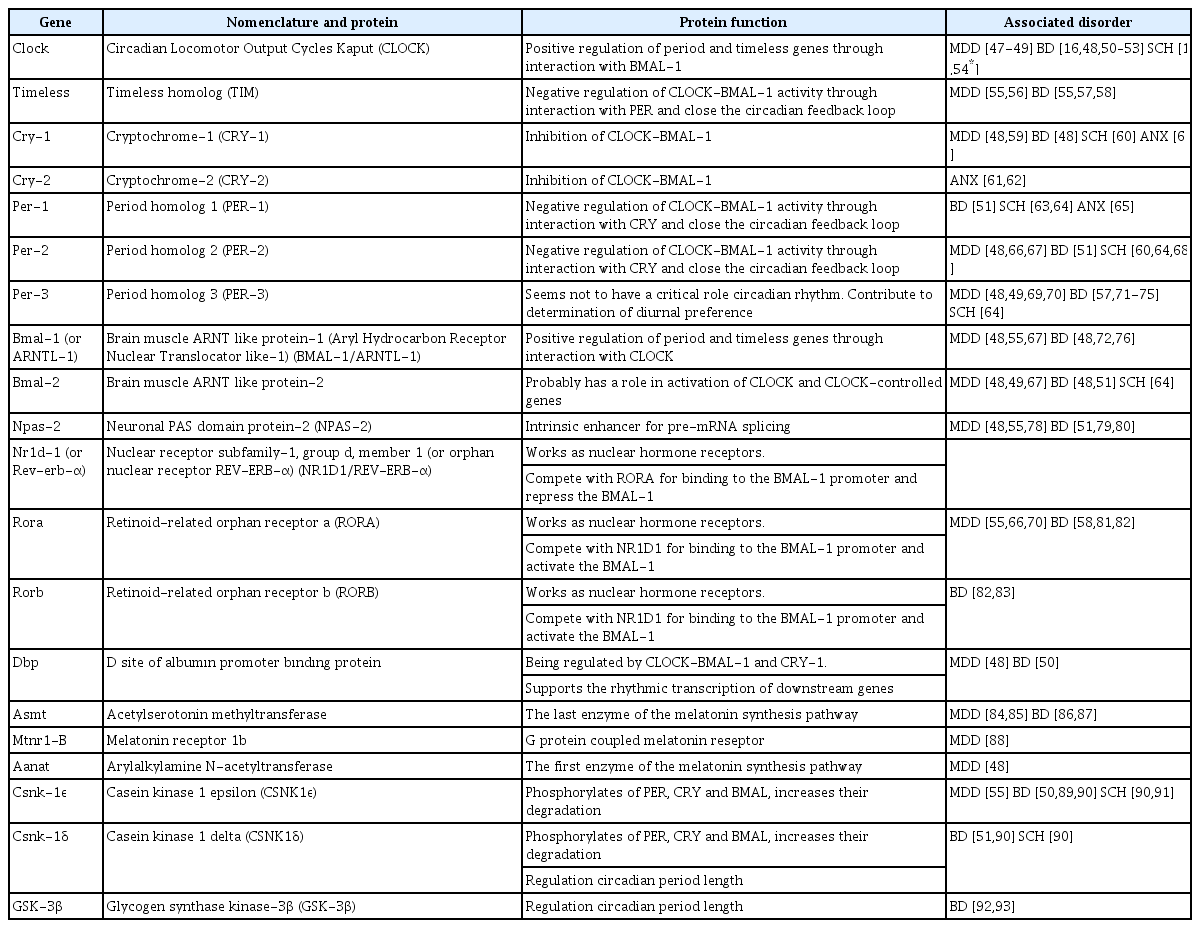
The master clock synchronizes the endogenous rhythm to the external world, mainly in the presence of major environmental input-light [30-32]. A specialized tract, called the retino-hypothalamic tract, which starts from the retinal ganglion cells that include the essential photoreceptor pigment melanopsin, and terminating at the SCN. This tract aids upregulation of clock gene expression and increases neuronal activity in the SCN [33,34]. Nevertheless, functions of the SCN, such as synchronization by the light/dark cycle, do not only depend on this molecular mechanism. Many inputs of the SCN have been determined including melatonin, food intake, blood pressure, and physical activity [35-38]. In addition, the SCN receives non-photic timing inputs from the raphe nucleus, which means the serotoninergic system plays a substantial role in the regulation of circadian rhythm [39]. Furthermore, the SCN serves in the excretion of numerous neurotransmitters that interact with other hypothalamic structures, hence neuropeptidergic signaling maintains circadian rhythm of the SCN. Consequently, the biologic interactions between the brain and body are modulated by the SCN, which is critically involved in the organism’s adjustment to the environment through the impact of internal signals, which are mediated by hormonal rhythms, the autonomic nervous system, and external time indicators such as light and food intake [10]. The master clock regulates the endogenous rhythm in response to environmental inputs and dysfunction of the master clock could contribute to a wide range of illnesses including obesity, diabetes mellitus, autoimmune disorders, and particularly mental disorders [40-44]. Disruption that arises due to a misalignment between inner physiology and the external world or a clock gene polymorphism may facilitate the emergence of diseases, increased disease severity and worsened prognosis, and heightened risk for poor treatment outcomes [45,46]. Clock genes, their main functions and association with psychiatric disorders summarized in Table 1.
NEUROHUMORAL AND HORMONAL REGULATION OF CIRCADIAN RHYTHM
The SCN collects information about the endogenous clocks through nervous projections and peripheral hormones. The SCN’s monosynaptic outputs mainly target the pre-autonomic neurons of the paraventricular nucleus (PVN) in the hypothalamus. The SCN is directly involved in the hypothalamic output to the preganglionic parasympathetic regions of the brainstem and to sympathetic preganglionic motor neurons of the spinal cord [94-96]. These projections allow the SCN to command the rhythmic control of hormone release and metabolism of all visceral structures through parasympathetic and sympathetic outputs. It has been determined that the SCN could increase glucose production from the liver through the sympathetic output to the liver with its projections that reach to the PVN [97]. Similarly, the SCN could increase corticosterone secretion in the adrenal or support glucose uptake into the muscle cells via sympathetic activation [98-100]. Besides, hormonal signals predominantly controlled by the SCN have a critical role in the regulation of internal synchronization [27]. Internal synchronization is supplied by adrenal glucocorticoids, pineal melatonin, adipocyte-derived leptin, pancreatic insulin or stomach ghrelin induced by the SCN. Internal synchronization included many multi-synaptic neuronal pathways that modulate behavior. For example, leptin increases during food intake in rats, ghrelin increases following a fasting period, and adrenaline increases with locomotor activity [100-102].
Glucocorticoids are produced in the adrenal glands from cholesterol and rhythmically released at ultradian (pulsatile) and circadian (daily) scales. Glucocorticoid release peaks typically prior to the onset of physical activity and depends on the fluctuations of corticotropin (adrenocorticotropic hormone, ACTH), a polypeptide secreted from the anterior pituitary under the control of corticotropin-releasing hormone (CRH), during the day. Glucocorticoid levels are regulated by a complex interaction between the adrenal clock and sympathetic outputs from the PVN and SCN [103]. Furthermore, the daily variation of glucocorticoids is influenced by stressful life events that activate the HPA axis and the autonomous nervous system. Glucocorticoid rhythm has a crucial role in the regulation of other hormonal rhythms and peripheral oscillations of metabolic gene expressions in the cells of tissues such as liver and white adipose tissue [103].
On the other hand, adrenal glucocorticoids can modulate the synchronization of the master clock to light via serotonergic projections from the raphe nucleus [104]. Serotonergic neurons release serotonin in the presence of glucocorticoid and locomotor activity. Such neuronal activity ensures transmitting feedback to the SCN in order to sustain the functioning of the clock itself [105]. In other words, serotonergic projections stimulated by locomotor activity provide a re-synchronization of the SCN [106]. Furthermore, brain serotonin synthesis and catabolism have their own circadian rhythm, closely related to the SCN. Neuronal serotonin release in the SCN is provided in the absence of photic stimulation, and serotonin levels increase in the raphe nucleus after the beginning of the dark phase [107]. Tryptophan hydroxylase (TpH), the rate-limiting enzyme in the synthesis of serotonin, is one of the regulators of circadian rhythm in the raphe nucleus. It is known that TpH peaks during the dark phase, helping the interaction between the serotoninergic system and the SCN through the increment of serotonin levels [107]. Also, serotonergic neurotransmission alterations could cause phase shifts and changes in SCN activity affecting the phosphorylation of CLOCK proteins [108].
Melatonin, a member of the class of acetamides, is another hormone related to biologic rhythm. It is primarily released by the pineal gland, particularly at night. Melatonin release is adjusted by the length of night time and melatonin per se regulates the seasonality of energy metabolism and reproduction in photoperiodic species [109]. Melatonin secretion peaks a few hours before sleep or at the time of minimal vigilance propensity, and decreases as wakefulness approaches under normal conditions [110]. In contrast, core body temperature reaches the highest degree during the day and has a nocturnal decline related to the melatonin peak [111]. This inverse relationship between melatonin and core body temperature is organized by the SCN. The nocturnal release of melatonin is induced by the SCN input to the PVN noradrenergic (sympathetic) afferents to the pineal gland [100]. Melatonin accumulates sleep both by setting the SCN and inhibiting neural centers such as the locus coeruleus (LC) and raphe nuclei, which mediate arousal through the ventrolateral preoptic nucleus of the hypothalamus (VLPO). It has been determined that melatonin receptor agonists increase monoaminergic neuronal activity and contribute to the regulation of dopamine and 5-HT neurotransmission [112]. In other words, melatonin has a modulatory role on the monoaminergic activity by linking the circadian and monoamine systems. The SCN modulates the release of melatonin mainly through γ-aminobutyric acid (GABA) neurons that project from the SCN to the PVN [113]. The daylight in the morning and the bright light in the evening activate the SCN neurons that inhibit the same PVN neurons through GABAergic projections and cease the secretion melatonin [32]. The daily rhythm of melatonin has remarkable effects on the molecular clockworks of both the brain and body alongside regulating the sleep/wake cycle [114,115]. Melatonin receptors (MT1 and MT2) are mainly localized in the CNS but also have been detected beyond the CNS in a wide range of somatic cells [116]. This diversity could be interpreted as melatonin having an integrative role in the light-induced circadian rhythms controlled by the SCN in the whole organism.
CIRCADIAN RHYTHM AND ITS IMPLICATIONS ON PSYCHIATRIC DISORDERS
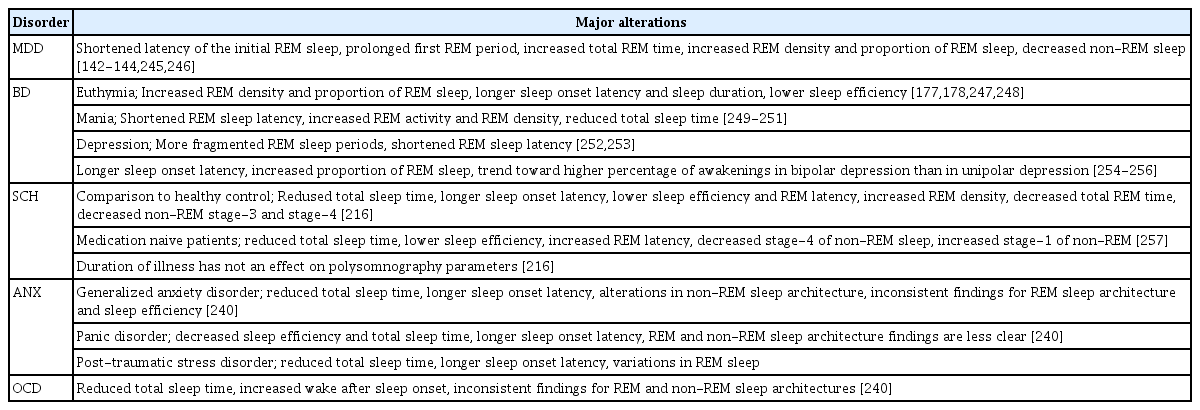
At the core of any psychiatric disorder is an abnormality in neurotransmitter signaling. It is well known that the disruption of circadian physiology has widespread effects on all aspects of neural and neuroendocrine function, which leads to psychiatric disorders. The aforementioned information regarding neural substrates of biologic rhythm is frequently reported impaired in many mental disorders. Following the comprehensive conceptual framework of neural substrates of chronobiologic processes mentioned above, we will next discuss the reciprocal associations between circadian rhythm disturbances and psychiatric disorders, and draw a clinical picture for common diagnoses. Main alterations of sleep architecture are illustrated in Table 2 and major consistent findings in the neurohumoral systems regulating circadian rhythm in psychiatric disorders are summarized in Table 3.
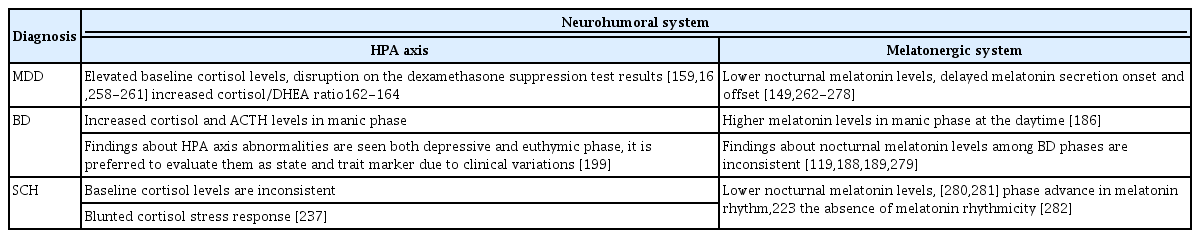
Summary of consistent findings on the alterations of two major neurohumoral systems regulating circadian rythm in psychiatric disorders
Mood disorders
In 1681, Robert Burton defined the autumn as the most melancholic season in his best-known classic, The Anatomy of Melancholia [117]. Circadian rhythm abnormalities in mood disorders have been pointed towards by the observers of melancholia for sixty years [118-120]. A wide range of body functions such as core body temperature, blood pressure, pulse rate, and hormones such as plasma cortisol levels, thyroid-stimulating hormone, and melatonin have been found disturbed in patients with manic depression and depression compared with people without a mental disorders [119,120]. Moreover, mood and other symptoms of the disorder have been previously reported to show diurnal variation in depression [121]. Disordered sleep/wake cycle is considered as another clue for physicians in patients with bipolar disorder (BD) and major depressive disorder (MDD) [121]. In addition, it was recognized that disrupted rhythms were re-synchronized after antidepressant or mood-stabilizing treatment [122]. Another significant feature is that mood episodes recur seasonally and previous studies showed that there could be an association between light and the emergence of mood states [123-127]. Thus, all of these findings suggested the possibility of circadian rhythm disturbance in mood disorders. Consequently, the earliest mention of seasonality took place in the Diagnostic and Statistical Manual of Mental Disorders Third Edition, Revised Version (DSM-III-R), and seasonal pattern was defined as a specifier in the affective disorders section [128].
Chronotype is another concept associated with mental disorders, particularly with affective disorders, and resembles individual physiologic functions and activities such as sleeping, eating, or hormone release. Chronotype has usually been used to denote sleep habits: morning and evening types. The relationship between chronotypes and several psychiatric disorders has been studied to date and the evening chronotype has been related to a vulnerability to depression and increased alcohol and stimulant drug use [129].
Although sleep/wake cycle alteration, which is considered as a consequence of circadian system disruption, had been the best-known contributor to the pathophysiology of mood disorders for years, today, it is well-recognized that circadian rhythm is entangled with a wide range of molecular and cellular processes that are hypothesized to lead to mood disorders [130]. Accordingly, below we discuss in detail internal and external factors that may play a role in the emergence of mood disorders through various psychophysiological mechanisms within the circadian rhythm processes.
Major depressive disorder
As a cardinal element of chronobiologic processes, sleep behavior and its disturbances have received the strongest spotlight regarding research into their undisputed etiologic and prognostic association with mood disorders. The concomitance of sleep disruption and depression had been the main focus of research into the contribution of circadian rhythms disruption to depression development since the 1970s [131-133]. The relationship between sleep and mood could easily be observed even in healthy individuals exposed to jet lag or shiftwork [134]. The presence of sleep disruption may cause negative effects, irritability, and fatigue. Sleep behavior changes, such as difficulties in initiating/maintaining sleep or early morning awakening have been determined in 90% of patients with MDD [18]. Sleepwake disruptions are among the criteria for the diagnosis of depression, and comorbid parasomnias are associated with poor treatment outcomes, increased suicidality, and greater relapse risk in depression [129,135-137]. Sleep architecture alterations including shortened latency of the initial rapid eye movement (REM) sleep, prolonged first REM period, increased total REM time, increased REM density and proportion of REM sleep, and decreased non-REM sleep have been demonstrated in depression [138-144]. It has been suggested that there is a reciprocal relationship between sleep-wake cycle variables such as wakefulness and sleep latency and sleep architecture features likes of REM sleep latency [145]. For instance, preclinical studies have consistently demonstrated that prolonged REM sleep was associated with decreased wakefulness in depressive subjects [146]. In addition, an endogenous circadian rhythm abnormality, the phase advance, is related to decreased REM latency after falling asleep among individuals with depression [147]. These findings suggest that sleep itself has multiple and complex regulators related with homeostatic mechanisms along with the endogenous circadian rhythm.
Melatonin output and the timing of its release have been found closely associated with other rhythms as mentioned above. Numerous studies have been conducted to show alterations of melatonin release and its phase to determine circadian misalignment between internal and external clocks in patients with mood disorders [148]. To date, the most consistent results suggested lower nocturnal melatonin levels, delayed melatonin secretion onset, and offset in patients with depression [148]. Besides, the length of the interval between melatonin secretion and sleep onset has been found related to depression severity [149]. However, a few studies demonstrated increased nocturnal melatonin levels in depressive patients [150]. Such conflicting findings might be related to the heterogeneity of the study group and inclusion of depressive patients with psychotic symptoms. Abnormal body temperature variations including the absence of the nocturnal decline of core body temperature and daily mean temperature degrees are also observed in patients with depression and these higher values normalized with antidepressant treatment [129,151]. This is probably due to the impaired control of the melatonergic system over thermoregulatory processes in depressive patients [151,152].
There is an irrefutable association between circadian genes and mood regulation. Even though mood disorders are not directly related to clock gene mutations, findings suggest that individual genetic polymorphisms of clock genes may influence the clinical features of the disorder, such as age at disease onset and treatment response [47,153]. Genetic studies have implicated clock, timeless, cryptochrome-1 (Cry-1), period-2,3 (Per2,3), Bmal-1,2, neuronal pas domain protein 2 (Npas-2), nuclear receptor subfamily-1, group d, member 1 (Nr1d-1), retinoid-related orphan receptor a (Rora), CSNK-Iε, D site of albumin promoter binding protein (Dbp), acetylserotonin methyltransferase (Asmt), melatonin receptor 1b (Mtnr1-B), arylalkylamine n-acetyltransferase (Aanat) genes in unipolar depression [48,66,154-156]. However, most of these studies have small sample sizes and need to be replicated in larger groups.
Glucocorticoids are adrenal steroid hormones and have multifunctional roles in the body and brain such as metabolism, immunity, arousal, neuronal survival, and neurogenesis [157]. Glucocorticoids have their own circadian rhythm and an important role in synchronizing peripheral clocks and the SCN. In addition, they have anti-inflammatory properties and regulate the immune system response [158]. Since Carroll defined the resistance of the dexamethasone suppression test in patients with depression in 1968 [159], hypothalamic-pituitary-adrenal (HPA) axis dysregulation has been one of the most consistent findings in mental disorders, particularly in depression [130,159]. Hypercortisolemia-flattened HPA axis circadian rhythm and disrupted response of the HPA axis to glucocorticoid feedback are commonly observed in patients with depression [160,161]. Dehydroepiandrosterone (DHEA), is another adrenal steroid that has a neuroprotective role and modulates corticosteroid-induced cell death. An increased cortisol/DHEA ratio, which assesses the degree of ‘functional’ hypercortisolemia, is seen in adults and adolescents with depression [162-164]. Glucocorticoid receptor hypofunction has also been found in peripheral tissue cells including mononuclear cells and skin cells [165]. Furthermore, findings support that antidepressant treatment repairs the impaired HPA axis dysfunction in depression [166].
Depression and inflammatory disorders such as rheumatoid arthritis, inflammatory bowel disease, and asthma have been found coexisting, and such common comorbidities point to the neuroinflammatory background and immune-associated contributions in the etiopathogenesis of depression [167,168]. Studies have also shown that pro-inflammatory cytokines could induce a depression-like symptom cluster including anhedonia, fatigue, increased sleep, and decreased locomotor activity [169]. Inflammatory markers such as interleukin (IL)-1β, IL-2, IL-6, tumor necrosis factor (TNF)-α, C-reactive protein (CRP), and prostaglandin E2 (PGE2) have been reported increased in patients with depression [170]. Sleep-wake cycle changes and circadian misalignment between the internal and external clocks may be other contributors to increased pro-inflammatory cytokine levels in depression. The arrhythmic clock system interacts with the nuclear factor-kappa B (NF-kB) signaling pathway, which is one of the major regulators of inflammation in the body and activates the inflammatory response [171,172]. Besides, sleep disturbances and long sleep duration were found related with the increased cytokines levels and the risk for depression [173]. We may interpret the aforementioned findings as the circadian system’s involvement in the pathophysiology of MDD being not limited to sleep/wake cycle disruption, it is also related to complex associations between biologic rhythm, environmentgene interactions, HPA axis dysfunction, and immune system alterations.
Bipolar disorder
Sleep disturbances have been the core common characteristic feature in bipolar mood episodes, both mania and depression, since the first definition of Kraepelin [174]. In turn, insomnia or hypersomnia and decreased need for sleep are typical for manic and depressive episodes. Studies showed that sleep architecture was characterized by increased REM density and reduced REM latency in bipolar manic episodes [175]. Sleep disturbances are also frequently observed in euthymic patients with BD. Increased REM density and the proportion of REM sleep have been shown in remitted patients with BD [176]. Moreover, findings revealed that remitted patients with BD have longer sleep latency and sleep duration and lower sleep efficiency [177,178]. Bipolar depression has similar polysomnographic findings including a tendency for more early awakenings and more fragmented REM sleep periods. However, total REM density was found greater in bipolar depression than in unipolar depression [176] (Table 2). Although abnormalities of sleep architecture are seen in episodes and inter-episodes, sleep disturbances worsen before relapses. Sleep loss and reduced sleep duration were defined as reliable predictors of hypomania and mania [176]. In addition, hypersomnia in euthymia is found associated with the development of upcoming depressive symptoms [179]. On the other hand, a large amount of euthymic patients describe symptoms that meet the diagnostic criteria for insomnia [178,180]. Sleep-wake disturbances have been found as one of the reasons for a worse course of illness, relapses, increased symptom severity, and poor treatment outcomes [181-184]. These findings may explain the reason for the treatment need in remitted patients with BD [136].
Involvement of the melatonergic system in the pathogenesis of BD through circadian dysregulations such as changes in the release timing, melatonin rhythm, and the sleep-wake cycle [176]. In terms of the phase relationship between the sleep-wake cycle and melatonin rhythm, it has been demonstrated that the melatonin secretion in the hours surrounding habitual sleep onset which is considered as a crucial circadian moment for sleep regulation was increased in patients with BD compared to those with unipolar depression [185]. Although findings of melatonin function in patients with BD are inconsistent, circadian system characteristics generally vary depending on the current episode; mania or depression [129]. Melatonin levels were found higher in the daytime in manic patients than in healthy controls and patients with depressive episode [186]. Findings about nocturnal melatonin levels among BD phases are not consistent [119,187-189]. It remains unclear as to whether these alterations derive from a primary dysfunction of the circadian rhythm or if they are secondary to sleep disturbances related to the BD episode. However, some studies supported the beneficial effect of exogenous melatonin administration, which provides sleep and mood improvement [190].
Some of the clock genes have been found intimately associated with both the onset of BD and illness course. Studies revealed that circadian gene polymorphisms may increase the predisposition to BD and indirectly affect recurrences and symptoms across all BD phases [191]. Genetic linkage and gene expression studies implicated the variant genes related to BD as clock, timeless, Cry-1, Npas-2, Bmal-1,2, Dbp, Nr1d-1, Per-2,3, Rora, Rorb, Asmt, Csnk-1ε, Csnk-1δ, and glycogen synthase kinase-3β (GSK-3β) [51,83,86,155,191,192]. It has been demonstrated that ClockD19, the mutant gene that occurs with the deletion of exon 19 in the Clock gene, produces a dominant negative CLOCK protein capable of DNA binding but deficient in transcriptional activity. This gene induces dopamine synthesis and increased dopaminergic activity, which result in an increase in tyrosine hydroxylase (TH) expression in the ventral tegmental area (VTA) and manic-like behavior in animal models [193-195]. Moreover, ClockD19-related higher dopaminergic activity in the VTA normalized after lithium treatment, which suggests increased dopaminergic activity may be the main reason for the manic-like behavior of mice [195]. Recently, several lines of evidence have emphasized the importance of the molecular and synaptic mechanisms of monoaminergic systems and circadian gene interactions, which are closely related to molecular alterations associated with the ClockD19 model in the VTA and nucleus accumbens [196]. On the other hand, lithium, a potent inhibitor of the GSK-3 enzyme, regulates the clock gene Nr1d-1 and BMAL-1 through GSK-3 [197]. Some polymorphisms including Clockrs3805148, Clockrs534654, Timelessrs11171856, and Timelessrs2291739 are associated with suicidal behavior in BD [198].
A dysfunctional HPA axis is suggested to play an important role in the pathophysiology of BD, although the mechanism needs to be elucidated. Increased levels of cortisol and ACTH are the most replicated findings in BD [199,200]. However, CRH levels are not determined to increase in BD [199]. Depressive symptoms and cognitive deficits are thought to be associated with the higher levels of cortisol, and ACTH and cortisol seem to be related to manic episodes [200]. A meta-analysis suggested that abnormalities of stress-related pathways including increased morning cortisol levels were mainly prominent in manic episodes. Such abnormalities are even observed in remitted patients, which means that the long-term pathology of the HPA axis is related to clinical states of BD and contributes to the stress-vulnerability models of illness development and progression [201].
Immune abnormalities have received increased attention due to their possible role in the pathophysiology of BD, as well as MDD. Systematic reviews on cytokine levels in patients with BD revealed that IL-4, IL-6, IL-10, soluble IL-2 receptor, soluble IL-6 receptor, and TNF-α levels were increased in patients compared with healthy controls, whereas IL-2, IL-8, IFN-gamma, and C-C motif ligand were not different from controls [202]. Moreover, a comparison of cytokine levels in another study determined that proinflammatory cytokines including IL-2, IL-4, IL-6 were higher during manic episodes, and IL-6 levels were higher in depressive state than in healthy controls [203]. It was also demonstrated that mood symptoms had a positive correlation with IL-6 and IL-2 levels [203]. When bipolar depression and unipolar depression were compared, sIL-6R, CRP, sTNF-R1, and monocyte chemoattractant protein-1 (MCP-1) were found at higher levels than in unipolar depression [204]. In conclusion, sleep disturbances may be a reliable indicator of an upcoming mood episode in BD.
Schizophrenia
Although the relationship between mood disorders and circadian abnormalities has become clearer in recent times, the links between schizophrenia and disrupted circadian rhythms have yet to be elucidated fully. However, sleep disorders and sleep-wake cycle alterations have been known as common and consistent features of schizophrenia and other psychotic disorders since the first definition of Kraepelin in 1883 [205]. Schizophrenia has been associated with abnormalities in sleep including delayed and advanced sleep onset, altered resting activity patterns, and irregular sleep-wake cycle [206]. Research into circadian abnormalities and sleep disruption in schizophrenia has attempted to explain the causal relationship in a reciprocal context. Hyperdopaminergia is a well-known phenomenon in psychosis syndromes and striatal hyperdopaminergic activity may be a result of sleep disruption and circadian abnormalities, and increased dopamine levels may induce sleep disruptions [207-209]. For instance, the Clock T3111C polymorphism, which is associated with increased dopamine levels in the SCN, has been determined in a population of Japanese patients with schizophrenia [14]. Furthermore, the blind-drunk mutant mouse, which carries a mutation in the gene encoding an exocytotic synaptic protein, synaptosomal-associated protein-25 (Snap-25), exhibits schizophrenia-like symptoms [210,211]. This mouse model of schizophrenia has been shown to display phase advance and fragmentation of the circadian cycle [212]. Most consistent findings of the circadian genetics studies have been associations between CLOCK, PERIOD1, PERIOD3, and TIMELESS genes and schizophrenia [213]. Circadian rhythms disruption has been reported in approximately 80% of patients with schizophrenia [214]. Abnormal sleep patterns in schizophrenia have been described in both unmedicated patients and patients currently receiving antipsychotic treatment [18]. The major findings in sleep architecture could be aligned, such as long sleep-onset latency, increased intermittent-awakenings, decreased total sleep time, and poor sleep efficiency [215]. Moreover, reductions in REM latency, REM density, and duration of non-REM Stage 4 are other alterations in micro-sleep architecture [17,18,216-218]. Sleep disturbances are also important to predict increased suicide attempts in patients with schizophrenia [219].
Melatonin is a versatile neurohormone that plays an important role in the pathophysiology of schizophrenia. 5-HT synthesis regulation, sleep-wake cycle, and anti-oxidant effects against neuroinflammation are impaired due to melatonin dysfunction in schizophrenia [208,220]. It has been shown that melatonin increases endogenous antioxidants by increasing phosphorylated glycogen synthase kinase-3 (GSK-3) levels and provides an anti-inflammatory effect [220,221]. Galván-Arrieta et al. [222] reported a reduction in axogenesis associated with lower levels of phosphorylated GSK-3 subtype β and less expression of melatonergic receptors in patients with schizophrenia compared with healthy controls. These findings may indicate a melatonin-derived neurodevelopmental deficit at a cellular level. A lack of normal melatonin rhythmicity, decreased nocturnal secretion of melatonin, and phase advance in melatonin circadian rhythms have also been described in patients with schizophrenia [208,220,223]. Additionally, pineal calcification in computed tomography has been demonstrated in patients with schizophrenia, and this structural change has been found associated with cortical atrophy [224]. Mainly, the clinical importance of the relationship schizophrenia and melatonergic dysfunction might come from the fact that impairment of sleep-wake cycles which are in close relationship with melatonin phase are associated with schizophrenia symptoms such as somatic complaints, anxiety, depression, and paranoia. Further support has shown that sleep-deprived schizophrenia patients might exhibit increased psychotic symptoms [208]. Moreover, the circadian rhythm of dopamine is dependent on melatonin [225]. A combination of sleep disruption and circadian rhythms disturbance may lead to the elevation of dopamine activity in the brain, both directly and through phase advance in melatonin circadian rhythm [208]. Preclinical studies have been also demonstrated that melatonergic receptor agonism may prevent the increase of glutamate release in prefrontal cortex [226], which has been suggested in the pathophysiology of schizophrenia, particularly of cognitive symptoms of the disorder [227]. Because of its significance in the pathogenesis of schizophrenia, melatonin has become a therapeutic target for researchers. It has been shown that melatonin agonists are efficacious agents for schizophrenia-associated sleep disorders and drug-related tardive dyskinesia [228,229]. Moreover, its improving effects on behavioral deficits via reducing brain oxidative stress have been shown in an animal model of schizophrenia [230].
The relationship between clock genes and schizophrenia is another undiscovered area for scientists. Few studies have been conducted to show linking circadian clock gene polymorphisms in schizophrenia to date. Takao et al. [14] identified the Clock 311C/T polymorphism, which is associated with higher dopaminergic neurotransmission in the SCN in patients with schizophrenia. These results were confirmed in another study conducted in a Chinese schizophrenic population [54]. Period-1 mRNA expression in the temporal lobe of post-mortem subjects with schizophrenia was found down-regulated when compared with healthy controls [63]. In addition, disrupted diurnal rhythms of the Per-1, Per-2, Per-3, Npas-2 and phase delay in the expression of Per-2 have been reported in white blood cells of patients with schizophrenia [64]. More recently, the absence of rhythmic expression of Cry-1 and Per-2 was determined in the fibroblasts of patients with schizophrenia compared with cells obtained from healthy controls [60]. Pinacho et al. [91] reported decreased levels of CSNK1ε protein levels in the prefrontal cortex of patients with schizophrenia. However, due to the small sample sizes of the available studies, the association between schizophrenia and clock genes still needs to be clarified with further studies with larger populations.
The stress-vulnerability model for schizophrenia was first proposed in the 1970s and has been further developed since that time [231,232]. Thus, the HPA axis has been one of the most attractive research targets to understand the pathophysiology of schizophrenia for decades. Increased cortisol levels have been determined in patients with schizophrenia and even in individuals at high risk for schizophrenia compared with controls [233-235]. However, mean baseline cortisol level measurements in schizophrenia are not consistent in the literature [236]. Nevertheless, blunted cortisol levels in response to stressors are much more consistent findings, regardless of disease stage, chronicity, and treatment condition [237]. To conclude, despite it being widely accepted that sleep and circadian disorders have an important role in the etiopathogenesis of schizophrenia, well-designed and comprehensive clinical studies are still needed to explicate the genetic and neurobiologic underpinnings.
Other psychiatric disorders
Anxiety disorders are seen as the most frequent type of psychiatric disorders with a lifetime prevalence of 29% in the general population [238]. Sleep disturbance is a common feature of anxiety disorders and is included in the symptom criteria for several anxiety disorders such as post-traumatic stress disorder and generalized anxiety disorder [239]. The presence of sleep disturbances has been reported as 74% in patients with anxiety disorders [176]. However, MDD as a frequent comorbid condition in anxiety disorders is a confounder in understanding the relationship of sleep disturbances and anxiety disorders. Studies related to generalized anxiety disorder have reported decreased total sleep time, increased sleep-onset latency, and alterations in non-REM sleep architecture, whereas findings of REM sleep and sleep efficiency are inconsistent [240]. Patients with panic disorder frequently have both sleep disorder and/or another anxiety disorder because they could have nocturnal panic attacks, which usually occur in Stage-2 or Stage-3 of non-REM sleep, as well as decreased sleep efficiency, total sleep time, and increased sleep onset latency [176,240]. Although sleep disturbances, including REM sleep-related nightmares, have been investigated in post-traumatic stress disorder, conclusions are not consistent [176]. There is no significant difference in sleep architecture in social anxiety disorder [241,242]. In an animal model, Cry-1 and Cry-2 gene protein deficiencies led to behavioral alterations characterized by an abnormally high level of anxiety [61]. Akiyama et al. [65] suggested that period-1 mRNA levels reduced after anti-anxiety treatment in the mouse cerebellum. Cry-2 expression was determined reduced in the hippocampus in another animal study [62]. Furthermore, a polymorphism in BMAL-2rs2306073 has been found associated with social phobia [77].
Obsessive-compulsive disorder (OCD) is another debilitating disorder that is segregated from the anxiety disorders category in the DSM-5 [243]. Although sleep disturbances have been reported including decreased total sleep time, alterations in REM and non-REM sleep architecture are less clear [240]. Certain chronotypes have been found as predictors of OCD symptoms in adults, and circadian rhythm disorders have been found as predictors of treatment outcomes [244]. To the best of our knowledge, the role of circadian rhythms disruption in all anxiety disorders, including OCD, has yet to go beyond showing sleep disturbance; comprehensive research is warranted in the context of chronobiologic mechanisms of anxiety disorder pathology.
CONCLUSION
The circadian system is responsible for the temporal organization of physiologic functions, and disruptions can have marked functional influences on the living organism. As the role of chronobiologic systems in both physical and mental health have become better understood, research into neurobiologic mechanisms of circadian rhythms has been expanded. Mood, cognition, and behavior have complex relationships with biologic rhythms, and the vast majority of mental disorders are reciprocally associated with impaired circadian biology. Extensive research has shown that impaired circadian mechanisms could lead to psychiatric entities, whereas they may be an outcome of mental disturbances. Impaired HPA axis function and melatonin homeostasis are the most consistent findings in mental disorders. Independent from sleep disorders, the circadian system has a distinct role in homeostatic processes, whose impairment has an impact in emotion regulation, cognition, behavior, and, most importantly, neural plasticity, all of which are often disrupted in psychiatric phenotypes. There is some evidence suggesting that circadian rhythm genes are associated with psychiatric disorders; however, the specificity and causality of these associations have yet to be made clear. In our opinion, we are a long way from establishing a robust causative link between circadian rhythms disruption and phenotypic complexity of psychiatric disorders. A decent translational approach to the findings of animal models would likely result in a clearer understanding of pathophysiologic implications of the circadian system. Further support from continued and integrated investigations of these issues may promote a deeper appreciation of the contribution of circadian disturbances to the pathophysiology of psychiatric illnesses and related psychopathology, and will hopefully yield improved therapeutic strategies for their treatment.
Acknowledgements
None.
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Simge Seren Kirlioglu, Yasin Hasan Balcioglu. Data curation: Simge Seren Kirlioglu. Methodology: Simge Seren Kirlioglu, Yasin Hasan Balcioglu. Supervision: Yasin Hasan Balcioglu. Visualization: Simge Seren Kirlioglu. Writing—original draft: Simge Seren Kirlioglu. Writing— review & editing: Yasin Hasan Balcioglu.