The Relationship Between Response-Inhibitory Event-Related Potentials and Symptoms of Attention-Deficit/Hyperactivity Disorder in Adult Patients with Major Depressive Disorder
Article information
Abstract
Objective
Attention-deficit and poor impulse control have frequently been observed in major depressive disorder (MDD) and attention-deficit and hyperactivity disorder (ADHD). Altered event-related potential (ERP) performance, such as GoNogo tasks, has been regarded as a neurocognitive process associated with attention and behavioral inhibition. The aim of this study was to investigate the association between Nogo ERP and adult ADHD in MDD.
Methods
A total of 64 participants with MDD (32 comorbid with ADHD) and 32 healthy controls aged 19–45 years were recruited; they performed GoNogo paradigms during electroencephalogram measurement. Beck Depression Inventory (BDI), State-Trait Anxiety Inventory (STAI), and the Adult ADHD Self-Report Scale (ASRS) were evaluated. Clinical measures and GoNogo ERP were compared between three groups: depression with ADHD, depression without ADHD, and healthy controls.
Results
MDD subjects with ADHD showed significantly decreased Nogo P3 amplitude at frontal electrode, compared with those without ADHD and healthy controls. MDD subjects with ADHD showed significantly longer Nogo N2 latency at frontal and frontocentral electrodes, compared with those without ADHD and healthy controls. In MDD subjects with ADHD, the Nogo P3 amplitude at the frontal electrode was negatively correlated with the ASRS score and inattention. The Nogo N2 latency at the frontal electrode was positively correlated with false alarm rate.
Conclusion
The decreased Nogo P3 amplitude in the frontal area might be a potential biological marker for inattention in depressed patients with ADHD.
INTRODUCTION
In general, 5–10% of men and 10–25% of women are diagnosed with major depressive disorder (MDD) at least once in their lives [1]. The main symptoms of MDD are depressed mood based on subjective complaints or others’ observation, loss of interest or pleasure, and decreased concentration. In addition to these mood symptoms, attention deficit [2,3], memory disturbance [4,5], decreased executive-control function [6,7], and poor response inhibition [8] are observed in MDD. Attention deficit and poor response inhibition are also known to be the main clinical features of attention deficit and hyperactivity disorder (ADHD) [9]. Each disorder shows symptom overlap, including attention deficits, emotional sensitivity, and poor impulse control [10]. Behavioral disturbances and response inhibition were commonly observed in patients with depressive disorder and ADHD [8,11].
Moreover, both MDD and ADHD show high rates of coexistence with each other. The prevalence of ADHD in MDD was reported to vary between 5 and 16% [12,13]. According to the National Comorbidity Survey Replication study [14], 18.6% of ADHD patients are diagnosed with MDD. In studies of adult diagnosed with ADHD, 35 to 50% of adults diagnosed with ADHD had more than one depressive episode in their lifetime [15-17]. In addition to higher levels of comorbidity, previous studies reported a genetic overlap between ADHD and MDD, while both disorders involve defective dopamine-reward circuit, and difficulty in emotional regulation [18].
When MDD coexists with adult ADHD, more severe symptoms and poor prognosis are predicted [19,20], which incur more cost to society and individuals [21,22]. Despite the importance of the detection of ADHD, psychiatrists have not been used to diagnose and treat adult ADHD, since ADHD was previously recognized as a childhood disease. In addition, ADHD is often overlooked in a clinical setting; psychiatrists are familiar with mood and anxiety symptoms, and they tend to diagnose mood or anxiety disorders in patients with ADHD symptoms [23]. To provide proper treatment for MDD patients with comorbid ADHD, biological markers that can differentiate MDD and comorbid ADHD should be detected.
The GoNogo event-related potential (ERP) is known to be a biological indicator that reflects behavioral and response inhibition [24]. Nogo N2 (N200) and P3 (P300) are well-known indicators of behavioral control [25]. Specifically, the Nogo N2 component appears to reflect inhibitory control or conflict monitoring [26], while Nogo P3 indicates motor inhibition [27-29]. The changes in Nogo N2 and P3 amplitudes have been investigated to compare the disease characteristics of inattention and poor impulse control in both MDD and ADHD; however, the results have been inconsistent. Many studies of MDD have reported increased Nogo N2 or decreased P3 amplitude in MDD patients compared to healthy control [30-33]. However, some studies showed no difference in Nogo N2 or P3 amplitude [30,31,33]. ADHD patients were generally found to show decreased P3 amplitude [34-38]. However, reports of change in the Nogo N2 amplitude in adult ADHD are inconsistent [34-36,39-42].
Although it is clinically important to diagnose ADHD in MDD patients, and despite a possible neurobiological basis for overlapping symptoms, such as inattention and behavioral inhibition, there have been no reports of neurophysiological differences according to the presence or absence of ADHD in MDD patients. Because N2 and P3 are known to be associated with behavioral inhibition, we hypothesized that Nogo N2 and P3 differ in patients with MDD, depending on the presence of ADHD. Thus, our aim was to investigate the association between Nogo ERP and adult ADHD in MDD patients.
METHODS
Subjects
All cases were between the ages of 19 and 45 years. We included 64 participants (37 male and 27 female, mean age=25.94±8.17 years) diagnosed with MDD according to the criteria of the Diagnostic and Statistical Manual of Mental Disorder, fifth edition (DSM-5). Bipolar disorder and other mood disorders were excluded via clinical interviews. In addition, a mood disorder questionnaire was administered and those who answered ‘yes’ to at least 7 of the manic or hypomanic symptoms were excluded [43]. MDD subjects were classified into either the ADHD or the comparison group. All patients diagnosed with adult ADHD met the full criteria for ADHD according to the DSM-5 criteria based on Mini- International Neuropsychiatric Interview by clinicians. We classified 32 subjects into MDD with ADHD group (27 males and 5 females, mean age=21.53±3.48 years), and 32 were classified into the MDD without ADHD group (10 males and 22 females, mean age=30.34±9.14 years). The healthy control group consisted of 32 physically and mentally healthy volunteers (13 males and 19 females, mean age=27.87±5.95 years) recruited from the local community through newspapers and posters. All patients with depression were drug-naïve. None of the participants had mental retardation, a history of substance abuse/dependence, or head trauma. This study was approved by the Institutional Review Board and Ethics Committee of Soonchunhyang University Cheonan Hospital, and all experimental protocols were approved by the committee (2019-05-004).
Clinical measures
To evaluate social and emotional functioning, we applied the Beck Depression Inventory (BDI), the State-Trait Anxiety Inventory (STAI), and the Korean version of the Adult ADHD Self-Report Scale (ASRS v1.1). The BDI is a self-reporting examination developed to measure depression. BDI consists of 21 items, with each item score ranging from 0 to 3, and the total score ranging from 0 to 63. The higher scores are positively correlated with the severity of depression [44]. The STAI is a selfreporting examination developed to measure two types of anxiety. The STAI consists of 40 items, with each item scored in the range of 1 to 4. Higher scores are positively correlated with a higher level of anxiety [45]. The Korean version of the Adult ADHD Self-Report Scale (ASRS v1.1) is a reliable and valid tool for screening and evaluating ADHD symptoms in adolescents and adults. The ASRS consists of 18 items (nine for inattention, and nine for hyperactivity/impulsivity). The answers that require careful evaluation are shaded in dark. Part A consists of six items. ADHD is predicted by four or more marks appearing in the dark-shaded boxes within Part A, warranting further examination. Part B consists of 12 items, and provides additional clues to the symptoms [46].
EEG data acquisition and analysis
Subjects were seated approximately 60 cm away from the computer screen in a relaxed sitting position in a silent room. EEG was acquired using a NeuroScan SynAmps amplifier (Compumedicus USA, E1 Paso, TX, USA) with 64 Ag/AgCl electrodes mounted on a Quik Cap. Electrodes were placed as central (Cz) and frontal (Fz), and an earth electrode was placed fronto-parietal (FPz), according to the extended 10–20 placement scheme. An electrode was placed infra-orbitally to monitor eye movement. Reference electrodes were placed at the mastoid, and the impedance was less than 10 kΩ. The bandpass filter was set at (0.1–100) Hz, and sampled at 1,000 Hz.
The EEG data were processed using CURRY 8. Gross artifacts were rejected by visual inspection by a trained person. Eye-movement artifacts were removed using the mathematical procedure in the preprocessing software. Data were filtered using a (0.1–30) Hz band-pass filter, and epoched from 100 ms pre-stimulus to 600 ms post-stimulus. These epochs were subtracted from the average value of the pre-stimulus interval for baseline correction. If any remaining epochs continued to have significant physiological artifacts (amplitude exceeding ±75 μV) in any of the 62 electrode sites, they were excluded from further analysis. Only artifact-free epochs were averaged across trials and participants for ERP analysis. Based on the previous studies that Nogo ERP reflected behavioral inhibition, the present study included Nogo trials in ERP analysis.
Behavioral task paradigm
As stimuli for the GoNogo task, we applied the ‘oddball paradigm’ of auditory stimulation. ERPs were elicited binaurally through headphones. The subjects were instructed to press the spacebar as accurately and quickly as possible when the target tone appeared, and not to respond when the non-target tone appeared. There were 400 trials, which consisted of Go (85% probability) and Nogo (15% probability) conditions. The target tone (Nogo) was 1,500 Hz, and the nontarget tone (Go) was 1,000 Hz, with a 1,500 ms interval before the next trial. These stimuli were generated using E-prime software (Psychology Software Tools, Pittsburgh, PA, USA). In the Go condition, N200 (the most negative peak between 150 and 350 ms after stimulus onset) and the P300 (the most positive peak between 250 and 500 ms after stimulus onset) were investigated at the frontal (Fz), fronto-central (FCz), and central (Cz) electrodes. In the Nogo condition, the N200 and the P300 were investigated at the Fz, FCz, and Cz electrodes. We focused on the changes of N2 and P3 at the frontocentral electrode, because MDD and ADHD have been regarded as mental illnesses associated with frontal-lobe dysfunction, and previous studies of MDD or ADHD patients generally showed changes of N2 and P3 in the fronto-central regions [30,31,34,39,47]. The time window we assumed was based on previous studies [48]. To accumulate behavioral data, Go accuracy, Nogo accuracy, and reaction time were calculated based on the data of E-prime software. The Nogo accuracy was calculated to determine the false-alarm rate of responses to non-target stimuli.
Statistical analysis
For descriptive statistics, we used frequency distributions, continuous variables, arithmetic means, and standard deviation. Groups were compared using the chi-square test for discontinuous variables. We used one-way analysis of variance (ANOVA) with a post hoc least significant difference (LSD) test to compare the scores of psychological and behavioral data between groups: the MDD with ADHD, MDD without ADHD, and healthy controls. N2, P3 amplitudes, and latencies of patients and healthy controls were initially evaluated by using repeated measures analysis of variance (ANOVA) with electrodes (Fz, FCz, and Cz) as the within-subject factor, and groups (MDD with ADHD, MDD without ADHD, and healthy controls) as the between-subject factor. The multivariate analysis of variance (MANOVA), repeated measures ANOVA were used to adjust for each of the variables including age, sex, education and all of them together as covariates. We used MANOVA to compare the GoNogo ERPs between MDD with and without ADHD groups, and control for age, sex, education and depressive symptom as covariates. In addition, Spearman’s correlation analysis was conducted between GoNogo ERP and psychological measures with a 5,000-bootstrap resampling technique to correct for multiple correlations. The bootstrap test is a weaker method than the Bonferroni test to solve the problem of multiple comparisons. However, the robustness and stability of the bootstrap test have been recognized by previous studies [49-51]. Furthermore, the bootstrap test has been widely used in EEG analysis [48]. We conducted a multiple regression analysis of Nogo P3 amplitude and psychological data that showed a significant association in the partial correlation analysis. Comparisons were considered significant at p<0.05. All statistical analysis was done using SPSS version 21.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Subjects
Table 1 presents the baseline demographic and clinical symptoms of MDD patients with and without ADHD, and those of healthy controls. The three groups differed significantly in age (p<0.001), sex (p<0.001), and education (p<0.001), based on by chi-square tests and analysis of variance (ANOVA) with a post hoc LSD test. The age of the patients diagnosed with ADHD was relatively low, because we targeted patients who received first-time examination at the hospital. The healthy control group showed significantly higher number of years of education than the MDD patient group (p<0.001). The MDD with ADHD group showed a significantly higher ASRS score than did the MDD without ADHD group (p<0.001). There were no significant differences in STAI state, STAT trait, or BDI between the MDD with ADHD group, and the MDD without ADHD group.
Behavioral outcomes
Table 2 presents the Nogo N2 and P3 behavioral outcomes. The MDD patients performed significantly worse in the GoNogo task on Go accuracy (MDD with ADHD, 93.31±8.81; MDD without ADHD, 95.40±7.07; healthy control, 99.75±0.62) and Nogo accuracy (MDD with ADHD, 85.37±14.07; MDD without ADHD. 90.56±9.96; healthy control, 97.31±4.47) compared with the healthy controls, who had significantly shorter reaction time than did the MDD patient group (MDD with ADHD, 476.02±102.76; MDD without ADHD, 458.80±86.27, healthy control, 414.92±68.27). There was no significant difference in reaction time between the MDD with ADHD and MDD without ADHD groups. The three groups of subjects showed significant differences in the false-alarm rate (MDD with ADHD, 14.62±14.07; MDD without ADHD, 9.43±9.96; healthy controls, 2.68±4.47). The healthy controls showed a significantly lower false-alarm rate than those with MDD with ADHD (p<0.001) and MDD without ADHD (p=0.010).
ERP
Amplitude
Table 3 presents the means and standard deviations for Nogo N2 and P3 amplitudes. Figure 1 shows the grand average of the Nogo ERPs at the Fz electrode for each group. The three groups showed significant differences in Nogo P3 amplitude at the frontal electrode (pre-adjusted, p=0.003; age-adjusted, p=0.007; sex-adjusted, p=0.001; education-adjusted, p=0.011; age, sex and education-adjusted, p=0.004). Also, compared with the healthy controls, the MDD with ADHD group showed a significantly lower Nogo P3 amplitude at the frontal electrode (pre-adjusted, p=0.001; age-adjusted, p=0.002; sex-adjusted, p<0.001; education-adjusted, p=0.003; age, sex and education-adjusted, p=0.001). However, the difference in Nogo P3 amplitude between group manifesting MDD with and without ADHD was significant only in sex-adjusted and total (including age, sex and education) adjusted values (sex-adjusted, p=0.013; age, sex and education-adjusted, p=0.036). When depressive symptoms were controlled by covariates within MDD patient groups, except for healthy controls, the difference in the Nogo P3 amplitude at the frontal electrode was not significant (p=0.067).

Comparison of the amplitude and latencies among MDD patients with and without ADHD and healthy controls
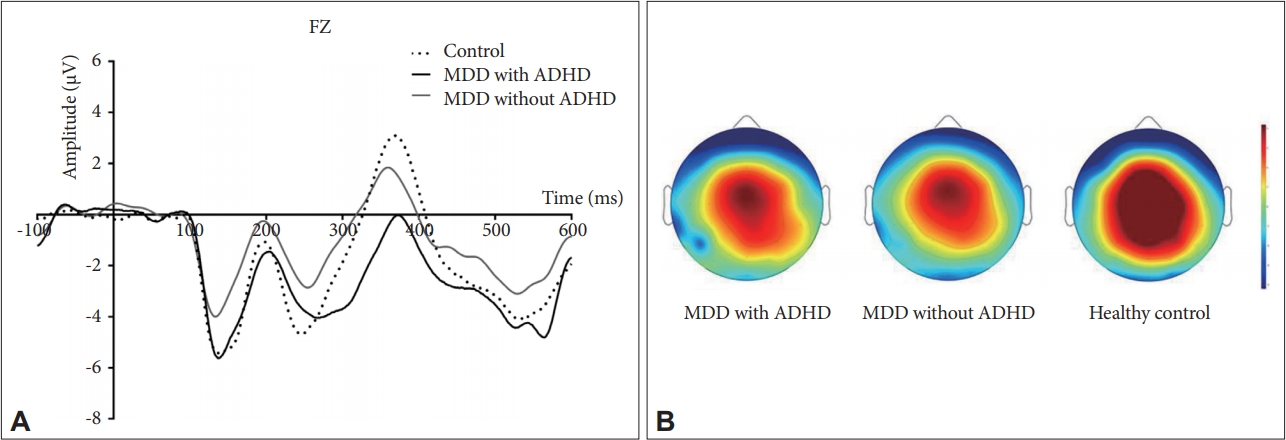
A: The grand average Nogo N2 and P3 waveforms at Fz in MDD patients with and without ADHD and healthy controls. B: Topoplot showing average activation across the scalp between (300 and 550) ms for MDD patients with for review only and without ADHD and healthy controls. MDD: major depressive disorder; ADHD: attention deficit and hyperactivity disorder, Fz: frontal electrode.
Latency
Table 3 displays the means and standard deviations for amplitude and latency of Nogo N2 and P3. Figure 1 shows the grand average of the Nogo ERPs at the Fz electrode for each group. The three groups showed significant differences in sexadjusted and total (including age, sex and education) adjusted Nogo N2 latency at the frontal (age-adjusted, p=0.026; age, sex and education-adjusted, p=0.047) and frontocentral electrode (age-adjusted, p=0.032; age, sex and education-adjusted, p=0.015). The MDD with ADHD group showed a significantly longer Nogo N2 latency at the frontal (age-adjusted, p=0.036; age, sex and education-adjusted, p=0.039) and frontocentral electrodes (age-adjusted, p=0.015; age, sex and education-adjusted, p=0.004) than did the MDD without ADHD group. The MDD with ADHD group showed significantly longer Nogo N2 latency at the frontal (age-adjusted, p=0.009; age, sex and education-adjusted, p=0.019) and frontocentral electrodes (age-adjusted, p=0.026; age, sex and education-adjusted, p= 0.050) than did the healthy controls. No significant differences in latency were found between the MDD without ADHD group and the healthy controls. When depressive symptoms were controlled by covariates within MDD patient groups, except for healthy controls, the difference in the Nogo N2 latency at the frontal (p=0.033) and frontocentral (p=0.009) electrodes was significant.
Correlations
In correlation analysis between the clinical symptom and ERPs measure in MDD with ADHD groups, the Nogo P3 amplitude at the frontal electrode was negatively correlated with the ASRS score (r=-0.358, p=0.044) (Figure 2A), and inattention (r=-0.438, p=0.012) (Figure 2B). Multiple regression analyses showed a significant association between Nogo P3 amplitude and inattention after adjusting age, sex and education. (B=-0.130, p=0.045). Nogo N2 latency at the frontal electrode was positively correlated with the false-alarm rate (r=0.371, p=0.037).
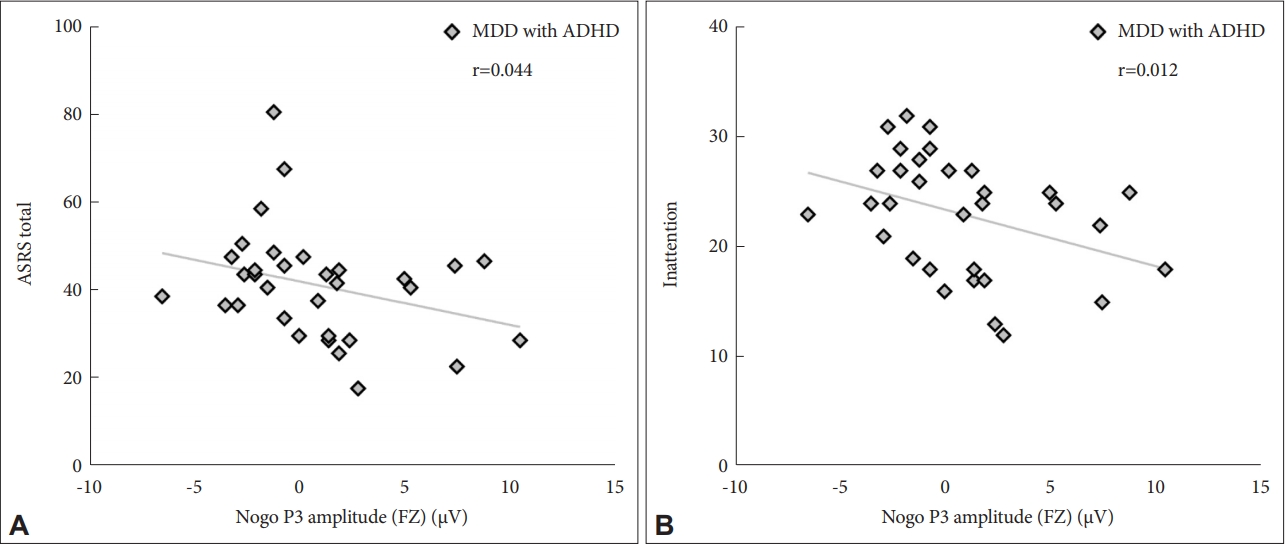
The Nogo P3 amplitude at Fz showed significant correlation with Adult ADHD self-report scales (ASRS) and the inattention subscale of ASRS. A: Scatter plots of Nogo P3 amplitude at Fz and total ASRS score change in MDD with ADHD patients. Spearman’s correlation analysis was conducted between GoNogo ERP and psychological measures. B: Scatter plots of Nogo P3 amplitude at Fz and inattention score change in MDD with ADHD patients. Spearman’s correlation analysis was conducted between GoNogo ERP and psychological measures. Fz: frontal electrode, MDD: major depressive disorder, ADHD: attention deficit and hyperactivity disorder, ASRS: The Korean version of the Adult ADHD Self-Report Scale.
DISCUSSION
Our study aimed to identify the differences of Nogo N2 and P3 ERP between the MDD with and without ADHD groups. As we hypothesized, the Nogo P3 amplitude varied according to the presence or absence of ADHD. The MDD with ADHD group showed significantly more decreased Nogo P3 amplitude at the frontal electrode compared with those without ADHD and the healthy controls. Additionally, the Nogo N2 latency also differed according to the presence or absence of ADHD. The MDD with ADHD group had a significantly longer Nogo N2 latency at the frontal and frontocentral electrode compared with those without ADHD and healthy controls.
First, the present study showed that Nogo P3 amplitude in the frontal electrode was significantly attenuated in the MDD with ADHD group relative to the MDD without ADHD and healthy controls. This finding was observed whether or not it was adjusted for age, sex and education as covariates. Although there has been no previous study that evaluated Nogo N2 and P3 in patients with MDD comorbid with ADHD, our results are comparable to the previous studies showing decreased Nogo P3 amplitude in MDD subjects compared to normal patients [30-32]. The previous study showed a significant negative correlation between depressive symptoms and Nogo P3 amplitudes [32]. Moreover, patients with ADHD also showed decreased P3 amplitudes, compared with healthy controls [34-38]. In particular, the inattention subscale in ASRS and P3 amplitude showed a significant negative correlation [37]. The present study also showed that Nogo P3 amplitudes were negatively correlated with inattention and ASRS score. Regarding P3 reflecting attention, working memory, and problem solving [26], our results indicate that the difference of Nogo P3 amplitudes between the MDD with and without ADHD might reflect the difference of attentional control between the two groups. In addition, the present study showed that MDD with ADHD had decreased Nogo P3 amplitude, compared with that of healthy controls. In contrast, no difference was found between the MDD without ADHD and healthy controls existed. Moreover, there was no significant correlation between Nogo P3 amplitude and depression symptoms, suggesting that Nogo P3 amplitude was related to inattention, rather than depression. It is possible that the results of previous MDD patient studies that showed significantly decreased Nogo P3 amplitudes might cause the characteristic of population including patients who had ADHD symptoms.
False-alarm rates and commission errors reflect poor motor-response inhibition, whereas reaction time and omission error reflect poor sustained and selective attention [52,53]. In behavioral tasks, MDD with ADHD patients showed increased omission rates and reaction times, compared to healthy controls in this study. The results are comparable to previous studies that found ADHD patients had a longer reaction time [38] and omission rate [52,54], compared to the healthy population. Additionally, regarding omission error reflecting poor sustained and selective attention [53], this result also suggests that ADHD patients showed poor attentional control. However, there was no significant difference in omission rate (Go accuracy) and reaction time based on comorbid ADHD in this study. These results suggest that omission error and reaction time do not reflect ADHD per se, but rather the attention deficit that appears in both MDD and ADHD.
Instead, the present study showed a significant difference in false-alarm rate and commission rate (Nogo accuracy) in MDD patients depending on the presence or absence of ADHD. Previous studies reported that ADHD patients had a higher falsealarm rate than did the healthy controls [36,38]. Our results suggest a possible difference in impulse control between MDD with and without ADHD patients.
Our results show that the Nogo P3 amplitude was correlated with the inattention scale of ASRS, but not with the falsealarm rate and commission rate, suggesting that the Nogo P3 amplitude reflects inattention, rather than poor impulse control. Nogo P3 amplitude might be a candidate marker reflecting the inattention characteristic of ADHD. Although the difference of Nogo P3 amplitude in the MDD group was not significant when depressive symptom was controlled, the multiple regression analysis showed a significant correlation between Nogo P3 amplitude and inattention. Further study is needed to corroborate the finding even after controlling clinical symptoms. The absence of differences in reaction time and omission rate between MDD with and without ADHD in the study might be attributed to the relatively easy GoNogo tasks, or the discrepancies between behavioral tasks and neurophysiological tasks. Also, due to the relatively small sample size, no possible correlation may exist between the clinical scale and the ERPs. Further study is needed to identify neurophysiological biomarkers associated with behavioral inhibition in the larger homogenous ADHD population with progressed behavioral tasks.
The present study showed that N2 latency in the frontal and frontocentral electrodes was longer in the MDD with ADHD group, than in the MDD without ADHD group. No previous Nogo N2 study of MDD with ADHD subjects has been reported. No study has reported significant changes in N2 latency in patient with MDD alone [30,31]. Moreover, few studies have reported changes in N2 latency among ADHD patients using the GoNogo paradigm, and no consistent findings have been reported [36,42]. Among the Nogo ERP studies involving patients with ADHD, Fallgatter et al. [36] reported that patients with ADHD showed longer N2 latency and longer reaction time compared with healthy controls. The previous studies using different paradigms have also reported a longer N2 latency in patients with ADHD than in healthy controls, which might reflect a lack of retrieval ability in the patients with ADHD [55,56]. In a study with childhood ADHD patients using the oddball paradigm, N2 latencies were longer in the frontocentral electrodes, and reaction times were longer than in healthy controls [56]. Additionally, in a study with adult ADHD patients using N-back task, the N2 latencies were longer in the occipital electrode, without any significant difference in reaction time [55].
Despite the lack of consistent findings related to N2 latency in patients with ADHD [47,57], in the study of Sunohara et al. [57], patients with ADHD showed shorter N2 latency, shorter reaction time, and higher false-alarm rate. These results suggest that patients with ADHD classified information more rapidly and in less detail compared with the controls.
The previous studies reported that the N2 component was associated with an unexpected degree of stimulation, along with response identification and selection [58,59]. Comparable to previous studies, a longer N2 latency in depressed patients with ADHD in this study suggests an additional cognitive process involved in classifying the stimulation [56]. Unexpectedly, there was no significant difference in reaction time between the MDD with ADHD and without ADHD groups. Furthermore, the Nogo N2 latencies were not correlated with reaction time, but with false-alarm rate. Considering the previous study that showed N2 in the GoNogo task was mainly associated with conflict monitoring processes [60], a longer N2 latency in depressed patients with ADHD might reflect more difficult conflict monitoring and additional cognitive load in processing the tasks. This would leads to weaker conflict monitoring, and causes more false alarm errors in the MDD patients with ADHD. Further study is needed to validate the inconsistent results reported previously due to differences in study populations and tasks.
This study has several limitations. First, the patient groups and healthy controls were not matched for age, sex, and education, despite adjustment for demographic variables as covariates. However, previous studies reported a generally higher rate of ADHD in males than in females from childhood into adulthood [61]. Patients with ADHD might be identified in early adulthood because of the nature of childhood onset and developmental pathophysiology, compared with those manifesting other psychiatric disorders. Although the discrepancy in age and sex between the study groups in this study might reflect clinical reality, a further study using a matched population is needed. Second, the findings are hard to generalize due to the relatively small sample size. Third, the subtypes of ADHD were not considered, and only DSM-5-based physician diagnosis and self-reported ASRS tests were used; which suggests the need for further studies using clinical diagnostic tools to corroborate our results. In addition, psychological scales measuring impulsivity, such as the Barrette impulsivity scale, were not evaluated in the study. Further study should consider direct psychological measurement of impulsivity. Finally, the present study did not investigate source activity of changed ERP of sensor level. Therefore, another study is needed for source analysis to confirm the role of prefrontal activity for attention and behavioral control in MDD with ADHD.
Despite these limitations, to the best of our knowledge, this study is the first of its kind to examine neurophysiological differences among patients with MDD according to the presence or absence of ADHD. Our study suggests that decreased Nogo P3 amplitude in the frontal area might be a candidate for a biological marker for inattention in ADHD co-morbid with MDD.
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2017R1D1A3B03030974). This study was also supported by Soonchunhyang University.
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: all authors. Data curation: Eun Jee Kim, Ji Sun Kim, Se-Hoon Shim. Formal analysis: Eun Jee Kim, Ji Sun Kim, Se-Hoon Shim. Funding acquisition: Ji Sun Kim, Se-Hoon Shim. Investigation: all authors. Methodology: all authors. Project administration: Ji Sun Kim, Se-Hoon Shim. Resources: Eun Jee Kim, Ji Sun Kim, Se-Hoon Shim. Softwear: Eun Jee Kim, Ji Sun Kim, Se-Hoon Shim. Supervision: Ji Sun Kim, Se-Hoon Shim. Validation: Ji Sun Kim, Se-Hoon Shim. Visualization: Eun Jee Kim, Ji Sun Kim, Se-Hoon Shim. Writing—original draft: all authors. Writing— review&editing : Eun Jee Kim, Ji Sun Kim, Se-Hoon Shim.


