Association between the IL10 rs1800896 Polymorphism and Tardive Dyskinesia in Schizophrenia
Article information
Abstract
Objective
Interleukin-10 (IL-10) is a major immunoregulatory cytokine and its gene plays a fundamental role in anti-inflammatory and immunosuppressive activity. This study aimed to examine the association between the IL10 gene promoter -1082G/A polymorphism (rs1800896) and tardive dyskinesia (TD) in schizophrenia.
Methods
Two hundred and eighty unrelated Korean schizophrenic patients participated in this study (105 TD and 175 non-TD patients). TD was diagnosed using the Research Diagnostic Criteria for TD and Abnormal Involuntary Movement Scale (AIMS). Genotyping was performed by RT-PCR and high-resolution melting curve analysis.
Results
The distributions of genotypic frequencies did not differ between patients with and without TD (χ2=4.33, p=0.115). However, allelic frequencies of the two groups were different (χ2=4.45, p=0.035); the A allele frequency was higher in TD. The total AIMS scores of the three genotypes were not different (F=1.33, p=0.266). However, the total AIMS scores of the A allele carrier and the A allele non-carrier were significantly different (t=5.79, p<0.001). Logistic regression analaysis showed that IL10 -1082G/A genotype significantly predicts presence of TD (p=0.045) after adjusting for covariates such as age and treatment duration.
Conclusion
This finding suggests that the A allele of rs1800896 may be associated with TD development following a low IL-10 function.
INTRODUCTION
Tardive dyskinesia (TD) is an involuntary, purposeless, and stereotypic movement disorder that develops in some parts of patients who have undergone long-term antipsychotic treatment. For typical antipsychotics, the accumulated prevalence of TD after 1-year treatment is 5%, and the prevalence increases by up to 20% after long-term treatment. The typical manifestations of this neuroleptic-induced disorder are as follows: 1) orofacial movements such as chewing, smacking of lips, protrusion of tongue [1], spasmodic blinking of eyes, and grimacing of the face and 2) choreic movements such as sudden and involuntary movements of the limbs. TD patients usually do not recognize these manifestations as serious adverse effects of antipsychotics; they may consider them as their odd habits and ignore them until they become severe. Therefore, psychiatrists need to investigate these symptoms thoroughly.
The etiopathogenic mechanism of TD is not well understood. The conventional hypothesis is that long-term administration of antipsychotics leads to dopamine receptor supersensitivity. However, the hypothesis does not adequately account for all cases of TD. First, although all rodents universally show dopamine supersensitivity following the administration of a dopamine receptor blocker, only 20–25% of patients who are treated with the first-generation antipsychotics have TD. Second, although older patients have decreased capacity for dopamine supersensitivity in their brains, the prevalence of TD increases with age. Third, TD symptoms are irreversible even after withdrawal from the antipsychotics [2]. Last, while it takes only several days for the number of dopamine binding sites to increase, it takes several months or years for TD to occur in patients who take antipsychotics [1].
Multiple line of evidence supposed that oxidative stress, imbalance between free radical and antioxidant, and resulting neurodegeneration is etiopathogenetic mechanism of TD [2]. Long-term exposure to antipsychotics increases dopamine turnover and leads to excessive production of oxidative metabolites [2,3]. Some studies have postulated that increased lipid peroxidation in the cerebrospinal fluid (CSF) due to imbalances in oxidation mechanisms can lead to cellular dysfunction and apoptosis of neuronal cells in vulnerable brain area such as dopamine-rich basal ganglia.
Old age, female gender, history of brain damage, high-dose antipsychotics, and long-term use of antipsychotics are known risk factors of TD. Excessive production of neurotoxic free radicals in patients treated with chronic antipsychotics exposure related, make vulnerable patient with low capacity eliminating excess free radical [1,4], have a degenerative change in vulnerable brain area (dopamine-rich basal ganglia) to develop tardive dyskinetia in the vulnerable period (old age). The effort finding genes carrying individual genetic susceptibility was focused in cytochrome P450 (CYP), neurotransmitters such as dopamine and serotonin, and oxidative stress [3,5].
IL-10 is an important immunoregulatory cytokine that acts on most hematopoietic cell types and enables various functions. IL-10 is also known to have anti-inflammatory and anti-oxidant effects. One of the most important effects is the decrease in TNF-α production to limit oxidative burst and alleviate oxidative stress [6]. IL-10 suppresses the inflammatory response via cytokines such as TNF-α and oxidative stress. Thus, it decreases the production of reactive oxygen species (ROS) [7]. Previous studies have shown that CSF IL-10 levels increase in schizophrenia patients and that IL10 gene polymorphism is associated with the susceptibility to, and the occurrence of, schizophrenia [8].
IL10 rs1800896, a single nucleotide polymorphism (SNP) located upstream of the IL10 gene, is the target of this study. According to a study in 2009, A allele of rs1800896 restricts the production of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-8 and increases the risk of prostate cancer [9]. Another study has shown that AA homozygotes of the promotor region in the IL10 gene reduces the production of IL-10. Therefore, reduced production of IL-10 due to AA homozygotes may prevent removal of increased oxidative stress, which makes patients treated with antipsychotics more vulnerable to TD.
Though the prevalence of TD may have decreased after the introduction of the second-generation antipsychotics [2], TD is still one of the most serious delayed, irreversible side effects of chronic antipsychotic use [10]. Thus primary prevention and early diagnosis of TD is crucial. To predict the occurrence of TD, this study investigated whether A allele of rs1800896 SNP prevents the production of IL-10, anti-oxidative function, and removal of oxidative stress, which in turn increases the occurrence of TD. This study also investigated whether the existence of the A allele in rs1800896 influences the AIMS scores.
METHODS
Subjects and assessments
A total of 280 unrelated Korean schizophrenia patients participated in this study. All the subjects were inpatients enrolled from the three collaborating hospitals of Korea University Hospital. They met the diagnostic criteria of schizophrenia, determined by board-certified psychiatrists using the Korean version of the Structured Clinical Interview for the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders. Subjects with significant neurological comorbidities, mental retardation, substance-related disorders, and other major psychiatric disorders were excluded. This study was approved by the Ethics Committee of the Korea University Hospital (06-2594), and all the participants provided written informed consent. Other characteristics of these subjects have been previously reported [11-15].
The participants comprised 105 and 175 schizophrenics with and without TD (non-TD), respectively, who were matched for exposure to antipsychotics and other relevant variables. All the non-TD patients had been treated with typical antipsychotics for at least 10 years. The TD sample included 25 patients who had taken atypical antipsychotic drugs and 18 who had been treated for <10 years. These differences between the inclusion criteria for the groups were acceptable as the occurrence of TD in patients exposed to antipsychotics for <10 years is highly indicative of genetic susceptibility.
All the subjects had taken a stable dosage of antipsychotics for >3 months before TD was assessed. TD was diagnosed based on scores on the Abnormal Involuntary Movement Scale (AIMS), which measures the severity of involuntary movements in seven domains on a scale from 0 to 4. Subjects were diagnosed with TD when they had two or more 2-point ratings or one or more 3-point ratings for the first seven items of AIMS. Psychopathology was assessed using the Positive and Negative Syndrome Scale (PANSS). Other findings related to these subjects have been reported previously.
Genotyping
Blood samples (5–10 mL) were collected in EDTA tubes, and genomic DNAs were isolated using a NucleoSpin Blood DNA Extraction Kit (Macherey-Nagel, Germany). The procedure followed the manufacturer’s instructions. Genotyping was performed using high-resolution melting curve analysis [16]. The polymerase chain reaction (PCR) was performed with a 20-μL reaction mixture in a 96-well CFX96 real-time PCR system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The reaction mixture included the following: 1) 2 μL of genomic DNA, 2) 200 mM of primer IL10 rs1800896, forward primer 5’-GAC AAC ACT ACT AAG GCT TCT-3’, and reverse primer 5’-ATG GAG GCT GGA TAG GAG-3’, 3) SsoFast EvaGreen Supermix (1×final concentration; BioRad Laboratories, Inc.), and 4) sterile H2O. The amplification protocol was as follows. An initial denaturation at 98°C for 3 min was followed by 39 cycles of denaturation at 98°C for 10 s and 58°C for 20 s. After the initial denaturation at 95°C for 10 s and 65°C for 10 s, melting curves were generated from 65°C to 95°C in increments of 0.2°C at each cycle. Melting profiles were analyzed with the Precision Melt Analysis software (BioRad Laboratories, Inc.).
Statistical analysis
The Hardy-Weinberg equilibrium was tested using the chisquare (χ2) test for goodness of fit. Categorical data were also analyzed using the χ2 test, and the differences between continuous variables were evaluated using the analysis of variance. Logistic regression analysis was used to determine if independent variable such as genotype of IL10 rs1800896 could account for TD (nominal dependent variable) after adjusting for confounding factors such as age, sex and medication duration. IL10 rs1800896 genotype was coded according to the number of G allele (AA=0, AG=1, GG=2) based on additive effect model. The analyses were performed using SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL, USA). All statistical analyses were two-tailed, and the level of statistical significance was set at p<0.05.
RESULTS
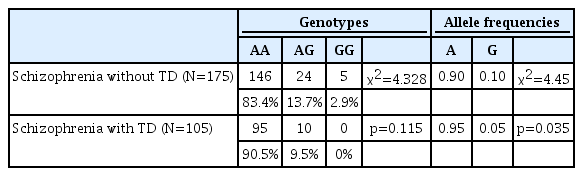
Of the 280 participants, 105 and 175 were included in the TD and non-TD groups, respectively. Table 1 shows the demographic data of the participants. Table 2 presents the genotypic and allelic frequencies of IL10 gene -1082G/A polymorphism. The distributions of the genotypic frequencies (χ2= 4.328, p=0.115) of TD and non-TD patients were similar. However, the allelic frequencies (χ2=4.45, p=0.035) of TD and non-TD patients demonstrated a statistically significant difference.
Comparison between the IL10-1082G/A (rs1800896) genotype and allele frequencies of schizophrenic patients with and without TD
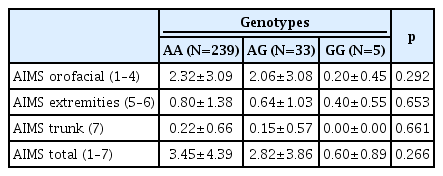
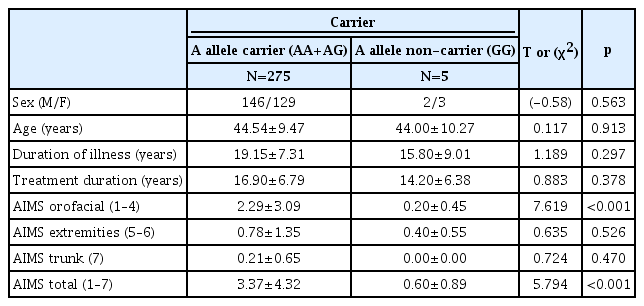
Table 3 shows the differences between the AIMS scores stratified by genotypes. There were no significant differences between the AIMS scores of the three genotype groups. Of the 280 subjects, 272 were A allele carriers (AA+AG) and 5 were non-carriers (GG). Table 4 shows the differences between the AIMS scores of the A allele carriers and non-carriers. The A allele carriers generally had higher AIMS scores than non-carriers; they had statistically significant higher orofacial (1–4), trunk (7), and total (1–7) AIMS scores, but not extremities (5–6) AIMS scores.
Comparison between the AIMS scores and clinical variables of the carriers of A allele (AA+AG) and the A allele non-carriers (GG) of IL10-1082G/A (rs1800896)
Although the sample size was small, there were fewer AG heterozygotes than AA homozygotes; the GG homozygotes were even fewer than AG heterozygotes. There was no GG homozygote among the schizophrenic patients with TD (Table 2). Sensitivity analysis performed by grouping AA vs. AG+GG showed no group difference of AIMS total score and subscores between AA and AG+GG groups.
We performed binary logistic regression analysis to elucidate whether IL10 -1082G/A genotype predict presence or absence of TD after adjusting for covariates such as age, sex and treatment duration. The model predicted that as individuals had more G alleles (AA=0, AG=1, GG=2), the likelihood to having TD significantly decreased (OR=0.473, p=0.045) (Table 5).
DISCUSSION
Several studies have emphasized on the association between genetic polymorphism and TD and found an association between TD and polymorphisms in genes related to 5-HT and dopamine receptors [5]. IL-10 is well known for its anti-inflammatory and anti-oxidant properties. Previous studies have reported that the SNP in IL10 reduces IL-10 production. There are data supporting the antipsychotic-related increase in oxidative stress in the brain and the relationship between oxidative stress and the pathogenesis of TD. The above-mentioned findings and data facilitated our study on the association between the etiopathogenic mechanisms of TD and rs1800896 of IL10.
IL-10 prevents TNF-α production and oxidative stress. The findings from our study support those of previous studies that the A allele of rs1800896 reduces IL-10 production and antiinflammatory and anti-oxidant reactions and inhibits alleviation of oxidative stress and neurotoxicity due to long-term administration of antipsychotics. This suggests that decreased IL-10 production causes critical damage to tissues, such as cardiac muscle or myelin sheath. Hence, the SNP of rs1800896 may reduce IL-10 production and increase oxidative stress and vulnerability to TD.
According to Capsoni et al. [17], IL-10 influences the inflammatory response of neutrophils. IL-10 inhibits oxidative burst by reducing lipopolysaccharide (LPS)-induced cytokine production of neutrophils, which suggests that A allele reduces IL-10 production, increases LPS-induced cytokine production from neutrophils, prevents removal of oxidative burst, and increases vulnerability to TD. As Table 2 demonstrates, the G allele reduces the susceptibility to schizophrenia, given that AG heterozygotes and GG homozygotes are markedly fewer than AA homozygotes. The absence of GG homozygotes in TD patients further underscores this postulation. However, this study is limited by the small sample size. If the sample was larger, statistically significant data may have been obtained.
Although long-term administration of atypical (second-generation) antipsychotics can also cause TD, typical antipsychotics are known to cause TD much more frequently. Therefore, atypical antipsychotics have become the primary drug of choice for schizophrenia. However, the efficacies of the two classes of antipsychotics are similar, and metabolic syndrome is a critical side effect of atypical antipsychotics. Furthermore, since typical antipsychotics are cheaper than their atypical counterparts, they are more widely used in clinical settings [5]. Therefore, it is important to predict high-risk groups who have genetically susceptible factors such as SNP; then, drug substitutions can be done before administration; otherwise, early diagnostic tools for TD are required urgently.
Small sample size and vicinity of GG genotype is limitation of our study. Extremely low frequency of G allele of IL-10 Sparsity of GG allele of IL10 rs1800896 in the east asian (GG genotype <1% of population) has limited the collection of sample sufficient to draw more conclusive conclusions. Further research is needed in other ethnics with a high frequency of GG alleles.
A previous study on the association between oxidative stress and TD highlighted that the antioxidant vitamin E (α-tocopherol) initially seemed to prevent and treat early phases of TD, but it showed low or no efficacy in longstanding TD. However, the accumulation of study data that support the association between oxidative stress caused by antipsychotics and TD provides the foundation for exploring the clinical application of antioxidants for treatment and prevention of TD [2]. In addition to the prevention of TD, modalities for preventing progression of TD and facilitating the recovery of patients are crucial.
Acknowledgements
This study was supported by the Korea Health 21 R&D Project funded by the National Research Foundation of Korea (2019R1A2C2084158 and 2017M3A9F1031220).
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Heon-Jeong Lee, Seung-Hyun Kim. Data curation: Jeong Min Choo, Youn-Jung Lee. Formal analysis: Kwang-Yeon Choi, Jeong Min Choo, Yujin Lee. Funding acquisition: Heon-Jeong Lee. Investigation: Kwang-Yeon Choi, Jeong Min Choo, Yujin Lee. Methodology: Kwang-Yeon Choi, Jeong Min Choo, Yujin Lee, Youn-Jung Lee. Project administration: Heon-Jeong Lee, Chul-Hyun Cho. Resources: Heon-Jeong Lee, Chul-Hyun Cho. Software: Kwang-Yeon Choi, Jeong Min Choo. Supervision: Heon-Jeong Lee, Seung-Hyun Kim. Validation: Heon-Jeong Lee, Seung-Hyun Kim. Visualization: Youn-Jung Lee. Writing—original draft: Kwang-Yeon Choi, Jeong Min Choo. Writing—review & editing: Heon-Jeong Lee.