Associations of Clozapine Use With Psychosocial Functioning and Quality of Life in Patients With Schizophrenia: A Community-Based Cross-Sectional Study
Article information
Abstract
Objective
More attempts have been made recently to improve psychosocial functioning and quality of life in patients with schizophrenia, due to their crucial role in long-term outcomes. Previous studies on the effects of clozapine on psychosocial functioning have been limited in terms of generalizability and application to clinical practice. This study examined the relationship of clozapine use with psychosocial functioning and quality of life in patients with schizophrenia in a real-world setting.
Methods
Data were obtained from a survey targeting community-dwelling patients with schizophrenia. The Behavior and Symptom Identification Scale (BASIS) and Satisfaction with Life Scale (SWLS) were administered to evaluate psychosocial functioning and quality of life, and patients were classified into Clozapine and Non-clozapine groups. Group differences were assessed using ANCOVA, with additional sensitivity analyses for participants on atypical antipsychotic medications only.
Results
Of 292 patients, the Clozapine group (n=34) had significantly better psychosocial functioning and quality of life than the Nonclozapine group (n=258), as demonstrated by their low BASIS score (F=4.651, df=1, 290, p=0.032) and high SWLS score (F=14.637, df=1, 290, p<0.001). Similar findings for psychosocial outcomes were observed in the analyses of the atypical antipsychotic subgroup (n=195).
Conclusion
For optimal recovery in schizophrenia, restoration of impaired social functioning and enhanced satisfaction with life are essential. In this study, clozapine use was related to high levels of psychosocial functioning and quality of life in real-world settings. Further research on the causal relationship between clozapine use and psychosocial functioning is needed.
INTRODUCTION
Impairment in everyday functioning is one of the core features of schizophrenia [1]. Thus, beyond simply achieving symptomatic remission, recent treatments have attempted to promote functional recovery in patients with schizophrenia [2,3]. As developments in treatment for schizophrenia have prolonged clinically stable periods and reduced recurrence, the effectiveness of antipsychotic medication should be evaluated not only in terms of the severity of psychiatric symptoms but also with regard to the level of psychosocial functioning or quality of life [4,5]. Targeting only positive or negative symptoms is not sufficient for achieving successful performance in interpersonal relationships and occupational functioning [4]. Furthermore, to predict long-term outcomes, it is crucial to assess a patient’s level of functioning in various social roles [6].
A comprehensive conceptualization of psychosocial functioning includes subjective wellbeing and quality of life as well as basic daily functioning, vocational functioning, interpersonal skills, and adaptive functioning [1,7,8]. Atypical antipsychotic drugs have emerged with improved efficacy and tolerability, but the effects of these atypical antipsychotics on the recovery of psychosocial function in schizophrenia are reportedly similar to those of conventional antipsychotic medication [9]. Clozapine is expected to have a greater impact on psychosocial functioning due to its beneficial effects on negative symptoms and cognitive function [10-13]. However, a recent meta-analysis reported that clozapine improved psychosocial functioning in patients with schizophrenia, but that its effects were not superior to those of other antipsychotics [7].
To comprehensively assess the level of psychosocial functioning in patients with schizophrenia, it is important to evaluate how actively patients engage in interpersonal relations and perform occupational functions in real-world situations [14,15]. However, most previous research examining the effects of clozapine on psychosocial functioning has taken place in inpatient or outpatient settings [9,16-19]. Furthermore, even community-based studies have had limitations such as a strictly controlled and standardized design that only compared antipsychotic monotherapy groups, which differs greatly from actual clinical practices [20].
Therefore, in this study we analyzed survey results from patients with schizophrenia residing in the community to assess real-world outcomes in terms of psychosocial functioning and quality of life. Seongnam Community Mental Health Welfare Center was established in 1999 and provides community-based mental health services to promote mental rehabilitation among citizens. In 2019, it surveyed the mental health condition and treatment status of all registered patients. We retrospectively analyzed data from this 2019 survey to explore the relationships of clozapine use with psychosocial functioning and quality of life.
METHODS
Study design
This was a community-based cross-sectional study using data obtained from the 2019 survey of registered clients of Seongnam Community Mental Health Welfare Center. Participation in the 2019 survey was voluntary, and patients who agreed to participate were asked to provide information and carry out several assessments. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital, Gyeonggi-do, Korea (IRB no. B-2012-654-108) and was conducted in accordance with the Helsinki Declaration of 1975 as revised in 2008.
Participants
Among the 997 patients registered in Seongnam Community Mental Health Welfare Center, 505 members replied to the survey. Study participants were selected according to the following inclusion and exclusion criteria. Patients diagnosed with schizophrenia or schizoaffective disorder and taking one or more oral antipsychotic medications were included in the study. Patients with comorbid major psychiatric disorders (e.g., major depressive disorder, bipolar disorder, mental retardation) or physical illnesses (e.g., cancer, cerebrovascular disease) were excluded from the study. Considering that psychosocial outcomes might be affected by whether antipsychotic medications were provided in an oral or a long-acting injectable formulation [21], patients using long-acting injectable antipsychotics were also excluded to allow assessment of the effectiveness of oral agents, as clozapine is not produced in an injectable form.
Participants who met the study criteria were classified into Clozapine and Non-clozapine groups based on whether they were taking clozapine. Among those in the Clozapine group, the Clozapine monotherapy group were those taking clozapine alone, and the Clozapine polypharmacy group were taking two or more antipsychotics including clozapine. The Nonclozapine group was also divided into monotherapy and polypharmacy groups, depending on the number of antipsychotics currently taken. Finally, the subgroup of patients taking only atypical antipsychotics was divided into Atypical Clozapine and Atypical Non-clozapine groups, based on whether clozapine was included.
Sociodemographic data and clinical information
Sociodemographic data included age, sex, education level, residential status, residential area, employment status, referral sources and duration of registration. Clinical information such as the age of onset, duration of illness, types and doses of antipsychotic medications, drug compliance, and drug side effects was also collected. Total doses of antipsychotic medication were calculated as chlorpromazine equivalents using formulas developed for previous studies [22-24]. Sociodemographic and clinical information was mainly collected through the self-reports of participants. The severity of psychiatric symptoms was examined using the Brief Psychiatric Rating Scale (BPRS) [25], which was administered by trained staff of the Seongnam Community Mental Health Welfare Center. The level of insight of each participant was also assessed by trained staff members.
Assessments of psychosocial functioning and quality of life
The Behavior and Symptom Identification Scale (BASIS) was used to evaluate major psychiatric symptoms and functional impairments reported by each patient [26]. BASIS is a self-reported questionnaire addressing the degree of neuropsychiatric symptoms and functional problems over the past week [26]. Scores for each item range from 0 (no difficulty) to 4 (extreme difficult) [26]. In this study, we applied the Korean version of BASIS [27], which consists of 31 items, excluding one school-related item from the original version of BASIS-32. Scores on the five subscales (relationship to self/others, daily living/role performance, depression/anxiety, impulsive/addictive behavior, and psychosis) and the overall average score were recorded [26].
The Satisfaction With Life Scale (SWLS) was also applied; this is a self-reported scale that assesses subjective quality of life [28,29]. Each of the five SWLS items is scored from 1 (strongly dissatisfied) to 7 (strongly satisfied); items are then summed to yield an overall SWLS score [28,29].
Statistical analyses
All statistical analyses were conducted using IBM SPSS Statistics, version 25.0 (IBM Corp., Armonk, NY, USA). The independent-sample t-test, chi-squared test, and Fisher’s exact test were used to compare demographic characteristics and clinical information among groups. Analysis of covariance (ANCOVA) was performed to examine significant differences in BASIS and SWLS scores between the Clozapine and Non-clozapine groups, adjusting for covariates. Comparisons among the Clozapine monotherapy, Clozapine polypharmacy, Nonclozapine monotherapy, and Non-clozapine polypharmacy group were analyzed using the Kruskal-Wallis test, followed by post hoc analysis using the Mann-Whitney test with Bonferroni’s method if significant differences were identified. The same analyses were conducted for patients who were taking only atypical antipsychotic drugs.
RESULTS
Sociodemographic and clinical characteristics
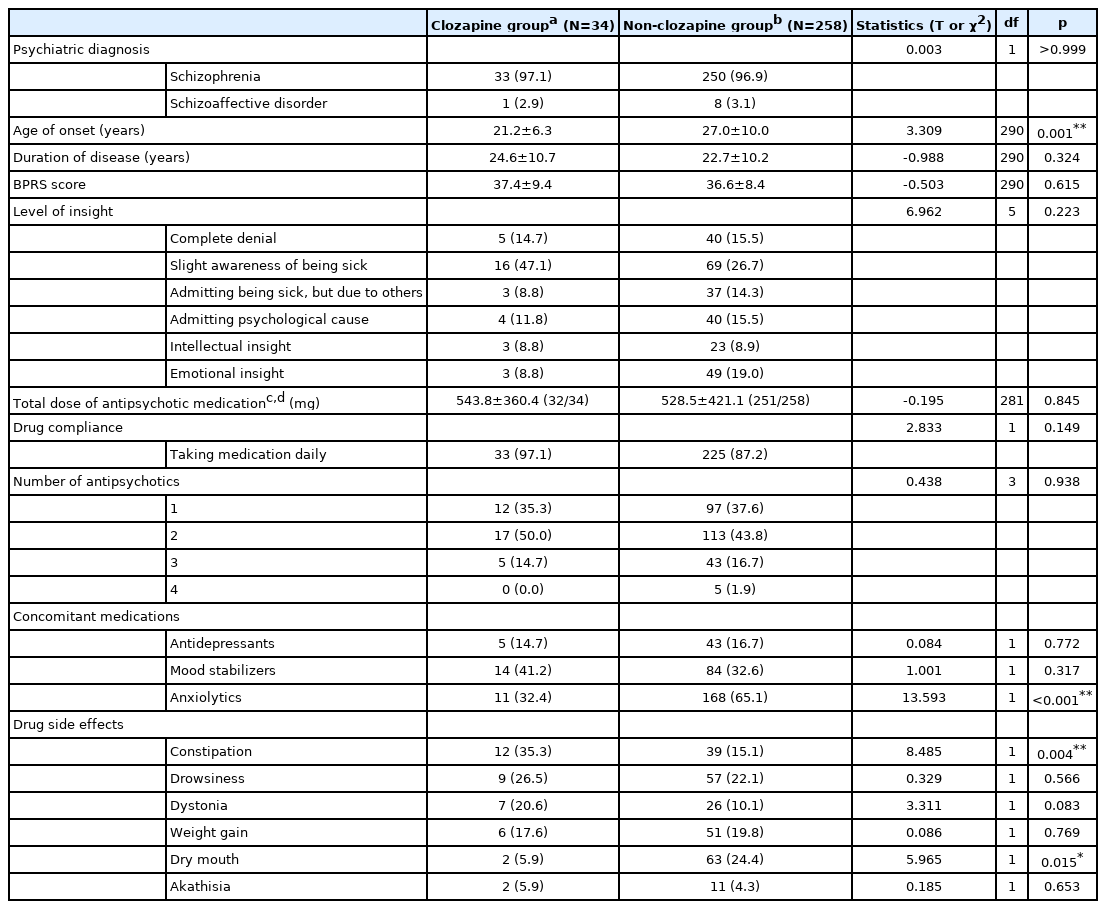
In total, 292 patients (283 with schizophrenia and 9 with schizoaffective disorder) met the criteria for study entry. Of these, 34 patients were classified into the Clozapine group, accounting for 11.6% of all participants. Table 1 lists sociodemographic data for the Clozapine and Non-clozapine groups. The mean age (±SD) of the Clozapine group was 44.6±11.8 years, which was significantly younger than Non-clozapine group, with a mean age of 48.7±10.7 years (t=2.039, df=290, p=0.042). More than half of the study population was male; the sex ratio did not differ significantly between the Clozapine and Non-clozapine groups (χ2=1.424, df=1, p=0.233). Education level, residential status, residential area, employment status and duration of registration were also comparable between the two groups. The distribution of referral sources was significantly different between the Clozapine and Non-clozapine groups (χ2=19.892, df=4, p=0.001).
Table 2 lists clinical data for study participants. The average age at onset in the Clozapine and Non-clozapine groups was 21.2±6.3 years and 27.0±10.0 years, respectively (t=3.309, df=290, p=0.001). The Clozapine and Non-clozapine groups did not differ significantly in psychiatric diagnosis, duration of disease, BPRS score, level of insight or total dose of antipsychotic medication. The rate of concomitant anxiolytic use was significantly higher in the Non-clozapine than in the Clozapine group (χ2=13.593, df=1, p<0.001), and the prescription rates for antidepressants and mood stabilizers were comparable between the groups (χ2=0.084, df=1, p=0.772; χ2=1.001, df=1, p=0.317, respectively).
Drug side effects frequently observed in the Clozapine group included constipation (35.3%), drowsiness (26.5%), dystonia (20.6%), and weight gain (17.6%). The proportion of participants who complained of constipation was significantly higher in the Clozapine group (χ2=8.485, df=1, p=0.004), whereas a significantly smaller percentage of participants reported dry mouth in the Clozapine group (χ2=5.965, df=1, p=0.015).
Comparisons of BASIS and SWLS scores among groups
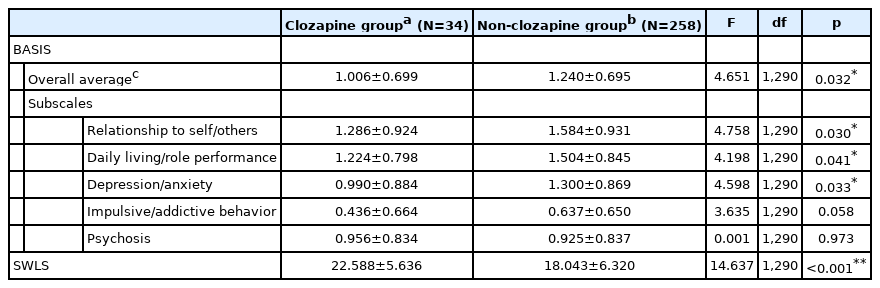
We performed ANCOVA to compare BASIS and SWLS scores between the Clozapine and Non-clozapine groups, adjusting for age and age of onset, which differed significantly between the two groups. As shown in Table 3, the overall average BASIS score was significantly lower in the Clozapine than in the Non-clozapine group (F=4.651, df=1, 290, p=0.032). The subscale scores for relationship to self/others, daily living/role performance, and depression/anxiety also differed significantly between the two groups (F=4.758, df=1, 290, p=0.030; F=4.198, df=1, 290, p=0.041; F=4.598, df=1, 290, p=0.033, respectively), indicating that patients in the Clozapine group had less functional difficulty in these areas. Furthermore, the SWLS score was significantly higher in the Clozapine than in the Non-clozapine group, indicating greater satisfaction with life among those in the Clozapine group (F=14.637, df=1, 290, p<0.001).
Figure 1 compares BASIS and SWLS scores among subgroups, including the Clozapine monotherapy, Clozapine polypharmacy, Non-clozapine monotherapy, and Non-clozapine polypharmacy groups. Notably, the Clozapine monotherapy group had the lowest BASIS overall score and the lowest scores on the five subscales, along with the highest SWLS score among the groups. For statistical analyses, the Kruskal-Wallis test was used, as the data were not normally distributed. Significant differences among groups were found for every BASIS and SWLS subscale score. Post hoc analysis with a multiple comparison correction revealed that the Clozapine monotherapy group had significantly lower scores compared to the Non-clozapine polypharmacy group for the BASIS overall average, relationship to self/others subscale, daily living/role performance subscale, depression/anxiety subscale, and impulsive/addictive behavior subscale (U=343.000, p<0.001; U=407.000, p=0.001; U=435.000, p=0.001; U=360.000, p<0.001; and U=418.000, p=0.001, respectively). In terms of SWLS scores, the Clozapine monotherapy group reported the highest level of satisfaction with life, although the scores for the Non-clozapine polypharmacy group differed significantly from those for the other three groups.
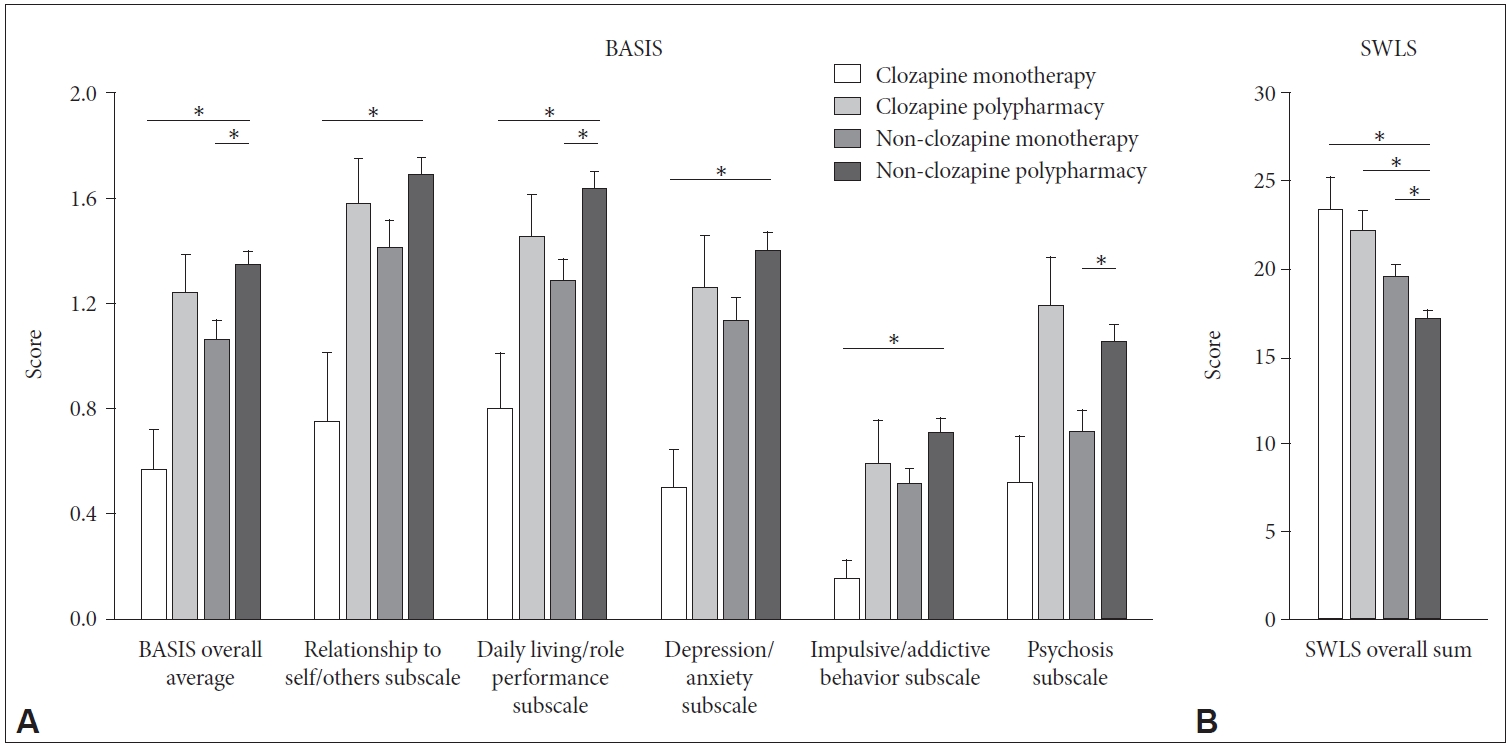
Comparisons of Behavior and Symptom Identification Scale (BASIS) and Satisfaction With Life Scale (SWLS) scores among subgroups. Each figure shows the results for multiple comparisons of (A) BASIS overall average and five subscale scores and (B) SWLS scores. Clozapine monotherapy group is a group of patients taking clozapine alone. Clozapine polypharmacy group consists of patients taking two or more antipsychotics including clozapine. Non-clozapine monotherapy group refers to patients taking only one antipsychotic medication other than clozapine. Non-clozapine polypharmacy group is composed of patients taking two or more antipsychotics other than clozapine. Each vertical bar indicates the standard error. Groups showing significantly different scores in post-hoc analysis with multiple comparison correction are indicated by horizontal bars. *p<0.05.
Comparisons of BASIS and SWLS scores between groups taking only atypical antipsychotics
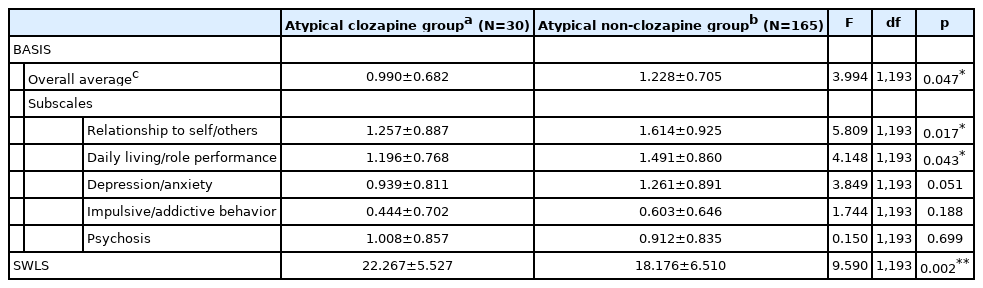
Table 4 lists the results of the ANCOVA analysis of the subgroup (n=195) taking only atypical antipsychotic medication, adjusting for the same covariates as above. The Atypical Clozapine group comprised 30 patients who took atypical antipsychotics including clozapine, and the Atypical Non-clozapine group consisted of 165 participants who took atypical antipsychotic drugs other than clozapine. The BASIS overall average score for the Atypical Clozapine group was significantly lower than that for the Atypical Non-clozapine group (F=3.994, df=1, 193, p=0.047). Among the subscales, significant differences between two groups were found for items addressing relationship to self/others (F=5.809, df=1, 193, p= 0.017) and daily living/role performance (F=4.148, df=1, 193, p=0.043). Moreover, the Atypical Clozapine group reported higher SWLS scores compared to the Atypical Non-clozapine group (F=9.590, df=1, 193, p=0.002).
DISCUSSION
This was the first cross-sectional observational study to evaluate the relationships between clozapine use and psychosocial functioning and life satisfaction under real-world conditions, including antipsychotic monotherapy and polypharmacy settings. The results revealed that clozapine use was significantly associated with better psychosocial functioning and quality of life in patients with schizophrenia. A similar relationship was found when we performed the same analyses on the subgroup composed of participants taking only atypical antipsychotics.
A prior meta-analysis reported that clozapine improved psychosocial functioning in patients with schizophrenia but that the effects were not superior to those of other antipsychotics [7]. However, several limitations hindered interpretation of the clinical implications of that study [7]. First, each study group included in the meta-analysis was defined as an antipsychotic monotherapy group in the studies covered [7]. However, the reported global median prevalence rate of antipsychotic polypharmacy is 19.6%, and that in Asia is even higher, at about 32% [30]. Considering that a substantial number of patients with schizophrenia are taking multiple antipsychotics concurrently, the results from previous research are not fully applicable to real-world clinical practice. An additional limitation is that studies conducted in inpatient/outpatient settings are not sufficient to understand an individual’s daily performance and functional outcomes in natural living environments [14,15]. Furthermore, the design of earlier research, which included participants recruited under controlled conditions, also limited evaluation of the effectiveness of antipsychotic treatments in the context of real-world outcomes [31-33]. The study populations in controlled trials hardly reflect real-world outcomes because only a small homogenous subgroup of patients is included in those studies, which use strict criteria and completely controlled conditions [31]. Indeed, it is well known that observational studies can be more helpful than controlled trials in estimating the real-world effectiveness of treatments or assessing long-term outcomes [31-33]. To achieve results reflecting actual daily environments, the present study explored data related to antipsychotic drug administration in real-world clinical practice with schizophrenia patients to assess quality of life and level of psychosocial functioning in community settings.
The results revealed that the onset of schizophrenia was significantly earlier in the Clozapine than in the Non-clozapine group. Furthermore, the Clozapine group had a longer disease duration and a higher proportion of males, although these differences were not statistically significant. These clinical characteristics of the Clozapine group are consistent with previous results characterizing treatment-resistant schizophrenia [34]. Furthermore, clozapine is recommended for treatment-resistant schizophrenia patients who do not respond well to other antipsychotic drugs [35,36]. Thus, the group classified as the Clozapine group in this study would likely represent patients with treatment-resistant schizophrenia, showing more severe symptoms and poorer treatment responses [34-36]. Based on previous research showing that the underlying pathophysiology of treatment-resistant schizophrenia differ fundamentally from treatment-responsive schizophrenia [37,38], some research groups have suggested that early administration of clozapine can be therapeutically beneficial even in cases of first-episode psychosis with insufficient response to other antipsychotic medications [39]. However, clozapine is often delayed in clinical practice due to problems such as drug-related side effects and concerns about periodic blood tests, which might be associated with quality of life [40]. Nonetheless, clozapine reportedly improves cognitive functioning and negative symptoms, which are also known to have serious effects on psychosocial functioning in patients with schizophrenia [10-13]. In this study, the Clozapine group had better psychosocial functioning and quality of life than the Non-clozapine group. Moreover, the Clozapine monotherapy group reported even higher levels of psychosocial functioning and subjective life satisfaction compared to other groups. These findings suggest that clozapine may be effective for functional recovery and improvement in life satisfaction in a selected group of patients with schizophrenia. Consequently, clinicians should consider using clozapine promptly and proactively in patients who fulfill specific indications.
Among the 292 patients in this study, 34 (11.64%) were taking clozapine. Xu et al. [41] reported that on average, 18.4% of patients with schizophrenia in Asian countries were taking clozapine, whereas the average rate of clozapine prescription in Korea was as low as 10.6%, which is similar to that identified in the present study. However, in practice, the proportion of treatment-resistant cases is estimated to be nearly one-third of all schizophrenia patients [42,43]. Approximately 40%–60% of patients with treatment-resistant schizophrenia are able to achieve clinical improvement through clozapine treatment [42,43]. In other words, the current status of clozapine use shown in this study suggests that the current rate of clozapine prescription is insufficient. Multidirectional approaches should be undertaken to overcome this problem by reducing improper delay in clozapine prescription.
This study had several limitations. First, analyses did not consider the effects of other drugs such as antidepressants, mood stabilizers, and anxiolytics. The Non-clozapine group had a significantly higher rate of concomitant use of anxiolytics, which may have had some adverse influences on patient functioning [44]. Additionally, the survey did not include information about the duration of drug use. According to Meltzer et al. [45], a treatment period of 6 to 12 months is required for clinical changes and functional improvement after starting clozapine. In this study, the effects of clozapine use may not have been fully reflected in the results, particularly for certain patients who just had started to take clozapine. Interestingly, clozapine use was significantly associated with higher social functioning and life satisfaction, even though the duration of clozapine administration was not considered in the study analysis.
Second, it was not possible to confirm causal relationships of clozapine use with psychosocial functioning and quality of life in patients with schizophrenia due to the cross-sectional design of the study. However, compared to those not receiving clozapine, patients taking clozapine might be more severely affected by schizophrenia considering that clozapine is currently recommended for treatment-resistant patients [35,36]. Furthermore, treatment with clozapine requires routine blood tests to monitor agranulocytosis and may have serious side effects [35,36]; this may reduce the effectiveness of clozapine in the domains of psychosocial functioning and quality of life. Given this context, it seems more reasonable to conclude that clozapine has contributed to the improvement of social functioning and quality of life in community settings, rather than to conclude that patients with higher levels of social functioning and life satisfaction would have been prescribed clozapine more frequently. Further research employing a longitudinal design is needed to verify the causal relationships between clozapine use and quality of life and psychosocial functioning.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Euitae Kim, a contributing editor of the Psychiatry Investigation, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions
Conceptualization: Euitae Kim. Data curation: Sujin Kim, Ah Young Choe. Formal analysis: Sujin Kim, Euitae Kim. Funding acquisition: Euitae Kim. Investigation: all authors. Methodology: all authors. Project administration: Seoyoung Kim, Euitae Kim. Resources: all authors. Software: Sujin Kim, Euitae Kim. Supervision: Seoyoung Kim, Euitae Kim. Validation: Seoyoung Kim, Euitae Kim. Visualization: Sujin Kim, Euitae Kim. Writing—original draft: Sujin Kim, Euitae Kim. Writing—review & editing: all authors.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korea government (MSIT) (No. NRF-2019R1A2C2005500, NRF-2019M3C7A1032472).