Development of the Korea-Polyenvironmental Risk Score for Psychosis
Article information
Abstract
Objective
Comprehensive understanding of polyenvironmental risk factors for the development of psychosis is important. Based on a review of related evidence, we developed the Korea Polyenvironmental Risk Score (K-PERS) for psychosis. We investigated whether the K-PERS can differentiate patients with schizophrenia spectrum disorders (SSDs) from healthy controls (HCs).
Methods
We reviewed existing tools for measuring polyenvironmental risk factors for psychosis, including the Maudsley Environmental Risk Score (ERS), polyenviromic risk score (PERS), and Psychosis Polyrisk Score (PPS). Using odds ratios and relative risks for Western studies and the “population proportion” (PP) of risk factors for Korean data, we developed the K-PERS, and compared the scores thereon between patients with SSDs and HCs. In addition, correlation was performed between the K-PERS and Positive and Negative Syndrome Scale (PANSS).
Results
We first constructed the “K-PERS-I,” comprising five factors based on the PPS, and then the “K-PERS-II” comprising six factors based on the ERS. The instruments accurately predicted participants’ status (case vs. control). In addition, the K-PERS-I and -II scores exhibited significant negative correlations with the negative symptom factor score of the PANSS.
Conclusion
The K-PERS is the first comprehensive tool developed based on PP data obtained from Korean studies that measures polyenvironmental risk factors for psychosis. Using pilot data, the K-PERS predicted patient status (SSD vs. HC). Further research is warranted to examine the relationship of K-PERS scores with clinical outcomes of psychosis and schizophrenia.
INTRODUCTION
Schizophrenia (SZ), one of the most detrimental and common psychiatric disorders, has an annual incidence of approximately 0.015% [1]. It often has a devastating impact on patients’ quality of life; about two-thirds of patients with SZ exhibit a chronic course characterized by relapse [2,3], and SZ patients have two- to threefold higher mortality compared to the general population, corresponding to a 10- to 25-year reduction in life expectancy [4]. The heritability of SZ, i.e., the genetic contribution to phenotypic variance, is high, ranging between 41% and 87% [5-7]. However, attempts to discover genes directly associated with SZ have been thwarted by a lack of replication of study findings. A recent genome-wide association study (GWAS) identified 108 loci associated with SZ [8]. These variants together explained only a small proportion (7%–36%) of the variance in liability [9], suggesting “missing heritability.” [10] Possible causes for the missing heritability of SZ include rare copy number variants, non-additive genetic effects (dominance and epistasis), gene–environment interactions, and environmental factors. Epidemiological studies have suggested that a variety of environmental factors, including prenatal infection/immune activation, paternal age, malnutrition, hypoxia-related obstetric complications, childhood/adolescence trauma and cannabis use are associated with an increased risk of developing SZ [11,12].
Given the small proportion of the variance explained by individual single nucleotide polymorphisms (SNPs), the polygenic risk score (PRS), a weighted sum of the number of risk alleles in individuals, is now considered as a valid alternative approach and has been widely applied in research studies. A similar approach has been employed to predict conversion to psychosis by aggregating environmental risk factors. Intervention prior to the full manifestation of a disorder may delay or even prevent the onset of psychosis; early identification of those at high risk of psychosis is thus of great importance. Three tools using the aggregate score for multiple environmental risk factors have been developed: the polyenviromic risk score (PERS) [13], Maudsley Environmental Risk Score (ERS) [14], and Psychosis Polyrisk Score (PPS) [15,16]. The environmental risk factors included in these tools differ. The PERS has nine risk factors (winter or spring birth, urbanicity, cannabis use, advanced paternal age, obstetric and perinatal complications, history of physical abuse, sexual abuse, neglect, and loss of a parent/parental separation), the ERS has six (ethnic minority status, urbanicity, high paternal age, obstetric complications, cannabis use, and childhood adversity), and the PPS has sixteen (childhood trauma, ethnicity, immigration, premorbid intelligence quotient (IQ), non-right handedness, olfactory identification ability, clinical high risk for psychosis, urbanicity, etc). Only the PERS score was shown to be significantly correlated with conversion to psychosis in individuals with familial high risk of SZ [13]. To apply these tools to populations in other countries with different cultures, the appropriateness of the included risk factors must be considered. The present study was undertaken to construct a Korean version of the PERS (K-PERS). After reviewing the evidence, we selected appropriate environmental risk factors for the K-PERS and calculated new risk scores based on the proportions of risk factors (exposure) in the Korea population. Pilot data from the K-PERS were compared between patients with schizophrenia spectrum disorders (SSDs) and healthy controls (HCs).
METHODS
Search strategy for relevant literature
Potentially relevant studies were identified by a comprehensive search of the PubMed, Embase, and PsychINFO electronic databases. Terms related to environmental risk in general, or to each putative risk factor (i.e., paternal age OR parental socioeconomic status [SES] OR pregnancy complication OR obstetric complications OR urbanicity OR child adversity/trauma/abuse OR cannabis/substance use OR recent life events) were combined with psychosis OR psychotic disorders OR SZ. The searches were limited to studies related to the ERS, PERS, and PPS, which measure multiple environmental risk factors, and to systematic reviews or meta-analyses of studies of putative risk factors. To determine the “population proportion” (PP) of risk factors in Korea, annual or survey reports issued by government-affiliated agencies (Ministry of Health and Welfare, Statistics Korea, Korea Land and Housing Corporation) were searched.
Development of the K-PERS
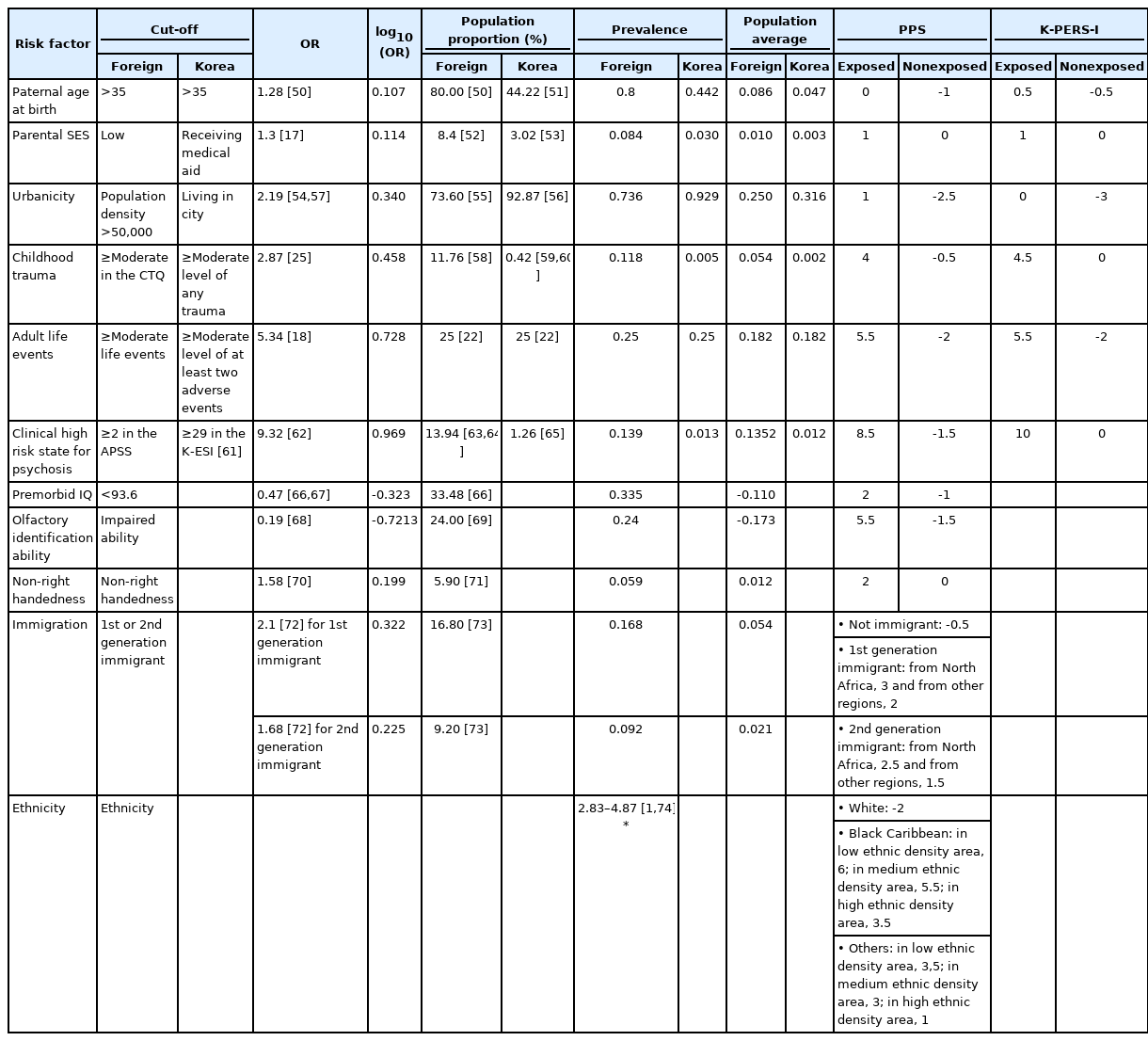
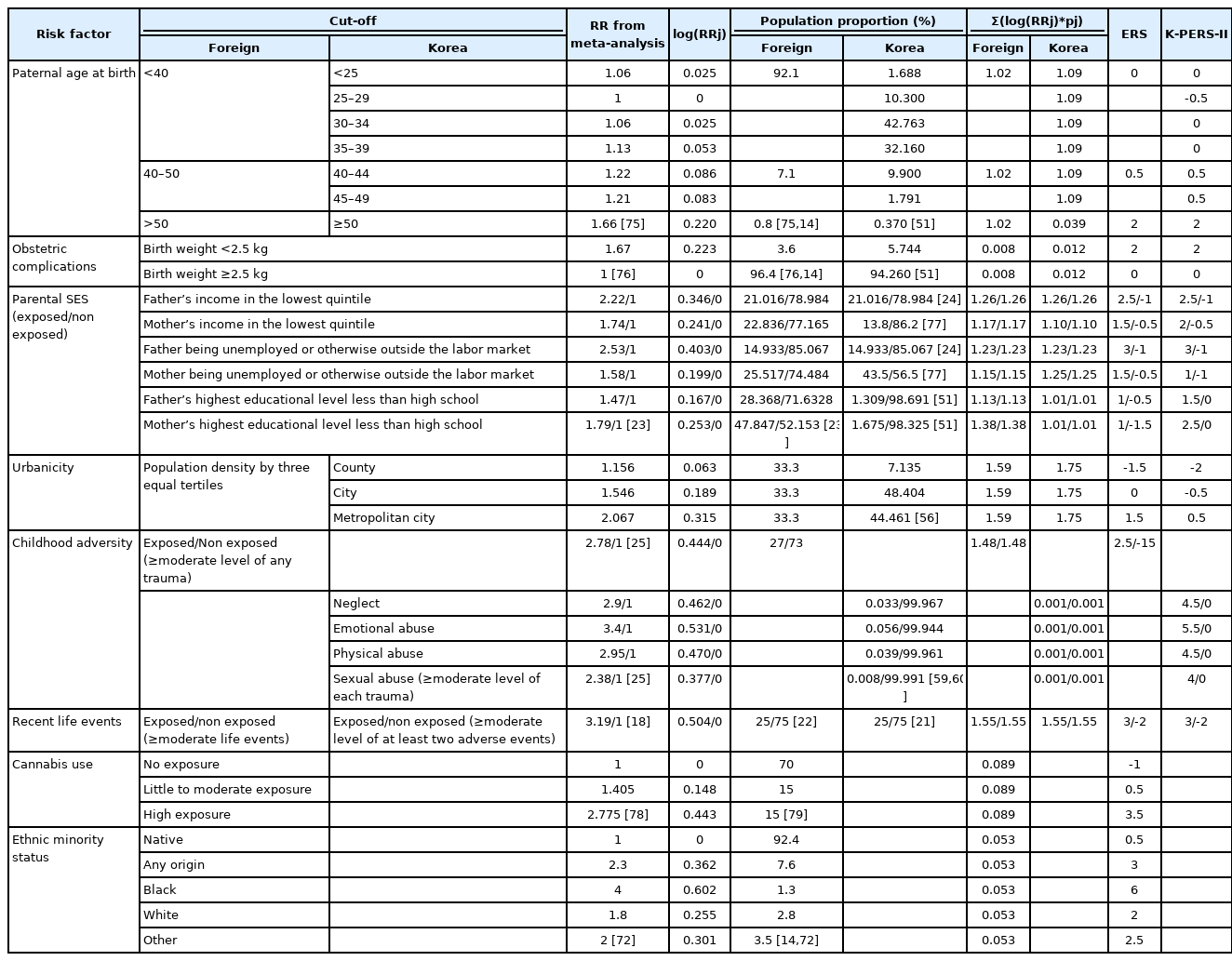
The ERS and PPS scores were estimated by scaling the odds ratios (ORs) or relative risks (RRs) with PP for each risk factor, whereas the PERS score was obtained by simply summing the ORs of the risk factors. We assumed that the former two tools may provide more valid estimates of the risk factors. Thus, we decided to consider the appropriateness only of the risk factors included in those two tools. Members of the Korea Early Psychosis Study (KEPS) team reviewed the risk factors included in the two tools. We decided that it would not yet be appropriate to include two factors, cannabis use and immigrant/ethnic minority status, in a tool designed for Koreans, although both factors are becoming increasingly important social issues in Korea. Also, premorbid IQ, olfactory identification ability, and pollution were considered to be impractical for clinical use. Paternal age, parental SES, and adult life events were not included in the original PPS [15], but were adopted for the extended version [16]. In the ERS, parental SES and recent life events were not included. However, we decided to include them given the associations of psychosis with social class [17] and life events [18], as well as their potential clinical utility. As we found no large-scale Korean epidemiologic studies investigating relationships between environmental risk factors and psychosis, we decided to use ORs and relative risks (RRs) from Western studies, and the PP of risk factors for Korean data, to devise the K-PERS. The formulas for calculating scores based on OR, RR and PP data are fully described in the original PPS [15] and ERS [14] publications. The K-PERS questionnaire (Supplementary Material in the online-only Data Supplement) was developed to evaluate six risk factors: paternal age, parental SES, obstetric complications, urbanicity, childhood trauma, and adult life events. Cutoffs were applied to some risk factors, such as paternal age, parental SES, birth weight, and urbanicity, whereas childhood trauma and adult life events rated as moderate or higher were assessed in terms of exposure. The K-PERS-I and -II were developed based on the PPS using OR and ERS using RR, because their subcategories of risk factors were different. In addition, considering importance of childhood trauma in the development of psychosis [14], subcategory and its cut-off of childhood trauma were differentially designed in the K-PERS-I and -II to reflect the data from self-rating scale and structured interview respectively. A manual for using the K-PERS was also developed and is available on request.
Pilot K-PERS data for patients with SSDs and HCs
Pilot K-PERS data were obtained from patients with SSDs, including SZ, schizoaffective disorder, schizophreniform disorder, and psychotic disorder not otherwise specified (n=130 and 217 for the K-PERS-I and -II, respectively), participating in the KEPS [19], and from HCs (n=126 and 154, respectively). HCs were recruited via advertisements and interviewed using the Structured Clinical Interview for DSM, Non-Patient Edition (SCID-NP) [20]. A requirement for study inclusion was no previous or current psychiatric disorders, neurological disorders, or significant medical conditions. Controls having a firstdegree relative with a psychiatric disorder were also excluded. The sample sizes differed according to the K-PERS version because we only included patients and HCs with complete data for the version used. The total and subdomain scores on the K-PERS were compared between patients and HCs. The relationships of K-PERS scores with duration of illness and Positive and Negative Syndrome Scale (PANSS) scores [21] were also examined. All participants provided written informed consent in accordance with a protocol approved by the Ethics Committee of Jeonbuk National University Hospital (approval number CUH 2014-11-002).
RESULTS
K-PERS-I
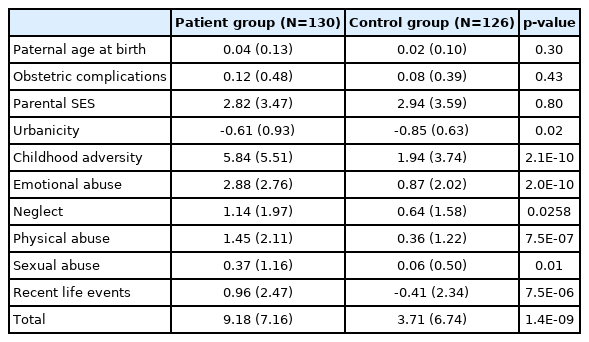
For the K-PERS, low parental SES was defined as receiving medical aid at the time of the respondent’s birth. Urbanicity was considered present when a person was raised in a city for more than 50% of their early life (from birth to 12 years old). Adult life events referred to at least two adverse events including living alone, financial hardship, and difficulties in social relationships and occupational or academic functioning, experienced at the age of ≥19 years at least 6 months prior to the development of psychotic symptoms. The PP values for paternal age, parental SES, urbanicity, childhood trauma, and clinical high risk for psychosis were acquired from official Korean data. However, as we did not find an appropriate data source for adult adverse life events, the same PP values used for the PPS [22] were applied. As the cutoffs and contents of the PPS are relatively simple, we named the developed tool the K-PERS-I (Table 1).
K-PERS-II
To accurately determine parental SES, a 7-point scale has been devised, on which low SES is reflected by on the presence of six factors (father’s/mother’s income in the lowest quintile, father/mother unemployed or outside the labor market, and father’s/mother’s highest educational level less than high school) [23]. As PP values were provided for each individual category but not for the summed score, six factors reflecting parental SES were included in the K-PERS-II. Because no Korean PP data for father’s income in the lowest quintile and father unemployed or outside the labor market were available, data from Byrne et al. [24] were adopted. Urbanicity was roughly categorized as “metropolitan city,” “city,” or “county,” as no Korean data on population density were available. In the ERS, childhood trauma was only categorized as exposed or nonexposed. However, considering its increasing importance in Korean society, more detailed categorizations [25] were used for the K-PERS-II. Although the references cited [18,22] in the PPS for recent life events are the same, using different RR values yielded different scores (3 and -2 for exposure and non-exposure) compared to those (5.5 and -2 for exposure and non-exposure) in the PPS. It is of note that parental SES and recent life events were not included in the original ERS. The tool developed based on the ERS was named the K-PERS-II (Table 2).
Comparison of K-PERS-I -II scores between patients with SSDs and HCs
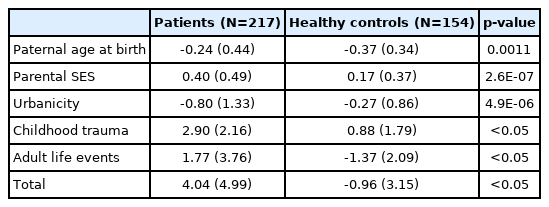
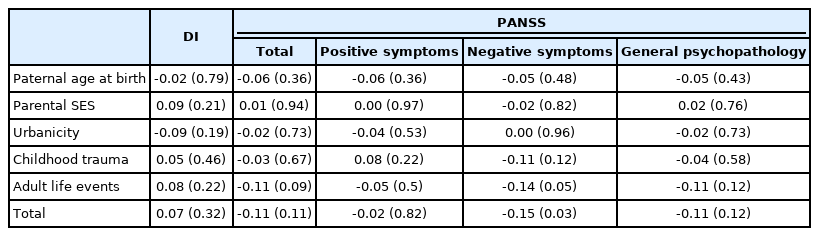
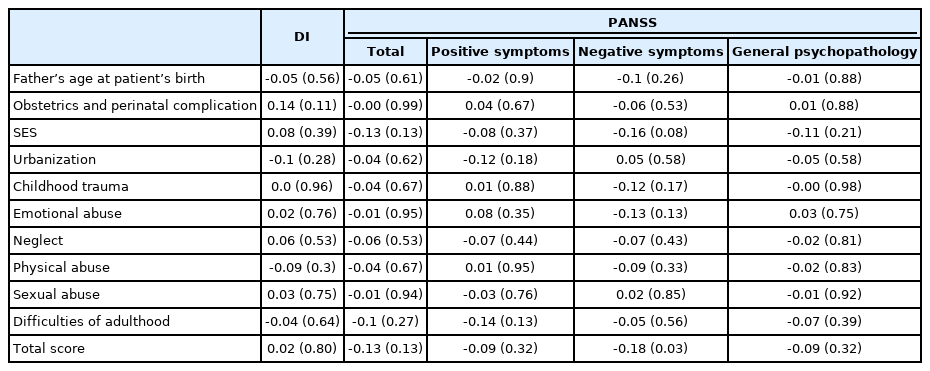
For the K-PERS-I, we only calculated the scores for the first five factors listed in Table 1 because the sixth factor, clinical high risk for psychosis, was not applicable. The demographic data (age, sex, and education) did not differ between patients and HCs according to the K-PERS-I and -II scores. The scores for all five factors of the K-PERS-I were significantly different between the two groups (Table 3). The total K-PERS-I and adult life events scores showed significant negative correlations with the negative symptom factor score of the PANSS (Table 4). For the K-PERS-II, only three factors were significantly different between the two groups (Table 5). The total K-PERS-II score exhibited a significant negative correlation with the negative symptom factor score of the PANSS (Table 6).
DISCUSSION
Among modern psychiatry disciplines, biological psychiatry has been the dominant research field, especially genetic and brain imaging studies. Most researchers in the field of molecular genetics now believe that many genes are involved (the polygenic theory) in the development of SZ, so have abandoned the single-gene approach [26,27]. Even the PRS, a measure based on a set of genetic variants as risk factors, has limited value for predicting SZ symptoms and cognitive phenotypes (<0.7%) [28]. Brain imaging studies of SZ have shown remarkable progress. Two major hypotheses developed based on these studies are that SZ is a neurodevelopmental disorder [29], and that it reflects disrupted brain connectivity (dysconnectivity hypothesis [30]. However, with regard to the clinical utility of structural MRI (sMRI), one meta-analysis found no evidence to support diagnosing SZ (as opposed to other psychotic disorders) based on the pattern of brain changes revealed by voxel-based morphometry (VBM) studies of patients with first-episode psychosis [31]. The specificity of altered functional connectivity (FC) to SZ is being questioned [32-34]. Furthermore, it should be noted that exercise [35,36] and meditation [37] can affect resting-state FC.
Numerous large-scale population-based studies have reported associations between various environmental factors and the prevalence of psychosis and psychotic symptoms [38]. The environmental factors include childhood adversity, poverty, migration stress, social isolation, social defeat, mother in poor health, early loss of parents due to death or abandonment, witnessing interparental violence, dysfunctional parenting (such as affectionless overcontrol and communication deviance), poor nutrition and stress during pregnancy, racism, and war trauma [39]. In contrast to the small amount of variance explained by genetic studies, the amount explained by environmental risk factors is quite high, at 33% of the estimated population attributable risk [25]. As in genetic studies, specificity regarding the roles of various environmental factors in the development of SZ remains a critical issue that has yet to be resolved; taking account of multiple environmental risk factors is important, similar to the PRS for genetic factors. The development of comprehensive measures assessing environmental factors in SZ is crucial for enhancing psychosocial understanding of the disease and obtaining holistic viewpoints from patients. In addition, it should be noted that some environmental factors are amenable to psychosocial intervention and education.
In the case of the PRS, many studies have explored its predictive value with respect to conversion in persons at clinical high risk [40], its discriminative ability in case (first-episode psychosis)–control studies [41], and its ability to predict antipsychotic efficacy in first-episode psychosis [42]. On the other hand, research using measures assessing polyenvironmental risk factors is limited. The PERS can predict conversion to psychosis in individuals with familial high risk of SZ [13]. In individuals with SZ, Stepniak et al. [43] reported an association between an increased number of environmental risk factors and age at SZ onset. Cougnard et al. [44] reported an interaction effect between exposure to three risk factors (cannabis use, childhood trauma, and urbanicity) and baseline psychotic experiences on the likelihood of psychotic symptoms 3 years later in the general population. To date, no study has investigated the relationships between polyenvironmental risk factors and clinical outcomes in SZ. In the present study, we found that total scores on the K-PERS-I and -II differentiated between patients with SSDs and HCs, suggesting their predictive utility for both patients with ambiguous clinical features and the general population. Of note, some items on the K-PERS-I and -II did not predict participants’ status (case vs. control), raising questions about their validity. As the cutoff criteria for factors in the K-PERS-II are more refined, it is recommended that it be used when detailed data are available. For example, when childhood adversity is measured with a self-rating scale, such as the Childhood Trauma Questionnaire (CTQ) [45] or Early Trauma Inventory—Self Report (ETI) [46], the K-PERS-I is recommended; the K-PERS-II is recommended when a structured interview is used. Correlation analyses identified significant negative associations of the K-PERS-I and -II scores with negative symptom factor scores. This suggests that multiple environmental risk factors may have an impact on the development of negative symptoms in SSDs. Given that negative symptoms are often viewed as having a neurobiological basis [47], this finding is meaningful, although the underlying mechanisms remain to be investigated.
Several limitations of this study should be mentioned. First, we decided to use OR and RR data from Western studies on relationships between polyenvironmental risk factors and SZ, so the K-PERS may not truly reflect Koreans’ experiences. To address this shortcoming, large-scale epidemiologic studies investigating relationships between environmental risk factors and psychosis should be performed. Second, ethnicity and immigration were not incorporated into the K-PERS. Due to increasing immigration from other Asian countries, this should be considered in future versions of the K-PERS. Third, conflict and stress related to family members was not considered in the K-PERS; however, this factor was also not included in the ERS, PERS, and PPS due to a lack of evidence. Nevertheless, several studies have suggested an important role of the family environment in the development of psychosis [48], and in poor prognosis [49]. When sufficient evidence accumulates, this factor will likely be considered as one of the main polyenvironmental risk factors for SZ. Lastly, to confirm validity of the K-PERS, future study with large sample size is warranted. Despite these caveats, the K-PERS is the first comprehensive tool developed based on PP data from Korean studies, and successfully measures polyenvironmental risk factors for psychosis. Using pilot data, the K-PERS was able to differentiate between patients with SSDs and HCs. Further research is warranted to examine the relationship of K-PERS scores with clinical outcomes in psychosis and SZ.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.30773/pi.2021.0328.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
Sung-Wan Kim and Euitae Kim, a contributing editor of the Psychiatry Investigation, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions
Conceptualization: Young-Chul Chung. Data curation: Yan-Hong Piao, Bong-Ju Lee, Sung-Wan Kim, Jung-Jin Kim. Formal analysis: Je-Chun Yu, Kyu-Young Lee, Seung-Hwan Lee. Funding acquisition: Young-Chul Chung, Seung-Hee Won. Investigation: Seung-Hyun Kim, Eui-Tae Kim. Methodology: Bong-Ju Lee, Clara Tammy Kim. Project administration: Young-Chul Chung. Resources: Shi-Hyun Kang. Software: Fatima Zahra Rami. Supervision: Young-Chul Chung. Validation: Paolo Fusar-Poli. Visualization: Dominic Oliver. Writing—original draft: Young-Chul Chung, Eun Jin Jeon. Writing—review & editing: Young-Chul Chung, Eun Jin Jeon.
Funding Statement
This study was supported by grants from the Korean Mental Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HL19C0015), and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (HI18C2383).