Incidence and Cognitive Decline of Alzheimer’s Disease and Other Dementia by Apolipoprotein ε4 Allele Presence: A Community-Based Cohort Study in Korean Elderly
Article information
Abstract
Objective
This study aimed to investigate the role of apolipoprotein E (APOE) ε4 allele to the incidence of dementia and cognitive decline in a cohort of a Korean community.
Methods
From a community-based dementia-free cohort, 357 participants were genotyped. Participants underwent 2 cognitive assessments separated by a hiatus between 6 to 7 years and were diagnosed as healthy control (n=297), Alzheimer’s disease (AD) (n=44), and other dementia (n=16) at the second assessment. Incidence risk and onset age of disease according to APOE ε4 presence were analyzed in AD and other dementia. Differences in cognitive decline rate depending on APOE ε4 were also examined across all groups.
Results
The relative risks and onset age of dementia were not different by the presence of the APOE ε4 allele. Cognitive decline was more prominent in the presence of APOE ε4 allele (score change=7.4) than non-presence (score change=3.1), and this interaction was significant only in the AD group (F=10.51, p=0.003).
Conclusion
The APOE ε4 alleles can be a critical factor in predicting cognitive change for AD in the Korean community population but not in predicting AD incidence. This finding suggest that clinicians consider the presence of APOE ε4 allele examining patients with rapid declining dementia.
INTRODUCTION
The Apolipoprotein E (APOE) ε4 allele is one of established risk factors for Alzheimer’s disease (AD) [1,2] and other dementia [3] and also associated with cognitive function. However, the prevalence of ε4 allele allele among AD patients vary by region and ethnicity [4].
APOE ε4 is also associated with an earlier age of disease onset from about 85 years without and ε4, 75 years with one, and 68 years with two ε4 allele even though this occurrence was not as pronounced in the male population [1,5]. The association between ε4 allele and AD prevalence is also reported in Korea [6]. This relation was also observed with AD patients older than 100 years [7].
Besides developing AD, the correlation of APOE ε4 with the rate of cognitive impairment, deterioration of function, and premature death have been reported in many studies [8-10]. Education and sex among the factors impacting on faster cognitive decline are considered to be associated with carriage of the APOE ε4 allele although the results are mixed still [11-13]. Ethoregional differences were observed also in the relationship between education and cognitive decline [14-16].
We aimed to investigate the effect of the APOE ε4 allele as longitudinal and lifetime risk of AD or other dementia and to investigate the relationship between education, sex and cognitive decline according to the APOE ε4 allele in risk across the Korean-community population.
METHODS
The study procedures were conducted in accordance with the Declaration of Helsinki and were approved by the Seoul National University Hospital Institutional Review Board, Seoul, Republic of Korea (IRB No. 0306-104-001). All participants received detailed information regarding the study aims and procedures. Written informed consent was obtained from all participants.
Participants
1,037 individuals who were legal residents of Yonchon Country and aged 65 years or older participated in the Korean Yonchon Survey (KYS) from December 1996 December 1996 through August 1997 (1st wave). Yonchon is an archetypical Korean rural agricultural county with a population of approximately 58,873 (female 26,748 [48.8%]) in 1996 (Statistical year book, 1996, Yonchon Gun). Random multistage cluster sampling was performed considering the population structure. Its methods and design are accurately illustrated in the KYS prevalence study [17]. Information was garnered on demographic variables and risk factors (age, sex, education, and literacy) during the screen phase through a semi-structured interview. Throughout the second phase, the diagnostic interviews were conducted on populations at risk and thus, a total of 370 subjects underwent phase 2 clinical evaluation, and this was conducted from January 1996 to August 1997.
A follow-up survey was conducted in 2000 and 2003 (2nd wave) for the incidence study. Total 357 individuals were included in this study for the population at risk excluding the 69 persons diagnosed with dementia n the initial survey. Following the consent of our subjects (n=357), blood samples were extracted to conduct APOE genotyping. Qualified psychiatrists performed clinical interviews as well as physical and neurological evaluations. The DSM-III-R, a criteria advocated by the Alzheimer’s Association or formerly established as the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA), served as the foundation for our clinical diagnosis of dementia and depressive disorder [18]. Probable or possible AD diagnosis were given to individuals who satisfied the criteria [19]. Subjects who satisfied the criteria recommended by the National Institute of Neurological Disorders and Stroke Association Internationale pour la Rechereche et l’Enseignement en Neuroscience (NINDS-AIREN) [20] were diagnosed as having probable or possible VaD. Participants were grouped as healthy control (n=297), AD (n=44), and other dementia (n=16) based on the results of the second assessment.
Korean Psychogeriatric Assessment Scale (PAS-K)
The Psychogeriatric Assessment Scale (PAS) is a multi-dimensional tool for the differential diagnosis of dementia and depression [21]. The Korean version of the Psychogeriatric Assessment Scale (PAS-K) has been shown to have good psychometric properties for screening for dementia and depression and have excellent validity and reliability on several parameters in Korea [22]. A cut-off score of 8/9 on the PAS-K Cognitive Impairment Scale has been shown to be sensitive in identifying potential cases of dementia. The increased PAS-K scores indicate cognitive decline.
Korean Dementia Rating Scale (K-DRS)
Developed by Mattis [23], Dementia Rating Scale (DRS) is a measure of the stage of cognitive impairment in demented patients. The Korean Dementia Rating Scale (K-DRS), a Korean version of the original DRS scale, was validated by Chey et al. [24]. The test comprises of five domains: attention, initiation and perseveration, visuospatial construction, conceptualization, and memory.
APOE type analysis
Genetic polymorphism was tested through PCR-RFLP (Polymerase Chain Reaction-Restriction Fragment Length Polymorphism). The DNA acquired from blood sample was amplified through the automatic thermal cycler (OmniGene, Hybaid Co, Middlesex, England) with the heat resistant Taq DNA polymerase (PerKinElmer, Waltham, MA, USA) and the properly biotinylated primer on the 5’ terminus. Following the denaturization of the amplified DNA through 5 minutes of denaturing solution exposure at room temperature, the nitrocellulose strip with immobilized sequence-specific oligonucleotide (SSO) probes was submerged, and the shaking water bath (Heto-Holten, Denmark) was fixed at 45°C and 80 rpm for 30 minutes to induce DNA hybridization. The color reaction in room temperature was induced using streptavidin with alkaline phosphatase markers, streptavidin of alkaline phosphatase, and substrate of alkaline phosphatase (5- bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium chloride). The APOE allele types were evaluated based on the read-out template.
The line formation for cysteine at position 112 identified ε3. Line formations for cysteine at position 112 and 158 specified ε2. Line formation for arginine at position 112 signified ε4.
Statistical analysis
Descriptive statistics were performed for APOE ε4 presence. Cox regression was conducted to analyze AD risk factor with APOE ε4 presence set as the independent variable and the onset age fixed as the observation period. A paired t test was employed to assess the mean difference between test values by dementia onset age. The interaction of APOE ε4 presence between groups (dementia, sex and education) and cognitive decline was analyzed through the General Linear Model Repeated Measures ANOVA. Statistical analyses were conducted using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY, USA). Statistical significance was based on 2-sided design-based tests evaluated at 0.05 significance level.
RESULTS
Demographic characteristics
At baseline, descriptive summaries of the study sample present means and standard deviations for continuous variables and frequencies for categorical variables (Table 1). 54.6% of the participants were women. The mean age and cognitive score in the initial wave were 71.6 years and 4.3, respectively. Age, education level, and cognitive score displayed statistically insignificant correlations with APOE ε4 presence. APOE ε4 allele frequency was 15.4% from the sample population, aligning with the worldwide average of 13.7% [25]. The APOE4 allele frequencies for AD and healthy control groups were 18.2% and 14.8%, respectively.
Dementia risk by APOE ε4 allele
Cox regression was performed to investigate the relationship between dementia onset and APOE ε4. The age, level of education, and sex of the non-dementia subjects from the 1st wave were corrected for covariates, the APOE variant was fixed as the independent variable, and the observation period was defined as onset age. While the presence of an APOE ε4 allele did not influence other dementia risks (hazard ratio, Exp(B)=0.746), the AD risks augmented by 1.05 (0.48–2.26) folds. However, this was statistically insignificant (p=0.906). The APOE ε4 carrier did not show statistically significant increases in both the AD and other dementia groups.
Dementia onset ages by APOE ε4 allele
The mean ages for APOE ε4 carriers and non-carriers were 71.4 and 71.6, respectively, suggesting that the difference between the two groups was not significant (p=0.837). The mean AD onset ages for APOE ε4 carriers and non-carriers were 78.5 and 79.2 (t=0.274, p=0.785). For other dementia, the mean onset ages for APOE ε4 carriers and non-carriers were 80.3 and 76.1 (t=1.554, p=0.143).
Relationship between APOE ε4 allele and cognitive decline rate across all participants
Our cohort investigation suggests that APOE ε4 is not correlated with AD, indicating that the allele may not be a pathological factor for the Korean demographic (p=0.587). However, APOE ε4 under AD displays statistical significance in accelerated AD progression through the augmented cognitive impairment score (Figure 1). For the AD group, the interaction between APOE ε4 carriers and ε4 non-carriers was significant (F=10.51, p=0.003, η2=0.221) and the main effect of time was also significant (F=63.68, p<0.001, η2=0.633). The mean cognitive impairment value for APOE ε4 carriers increased by 7.4 from 5.8 (1st wave) to 13.2 (2nd wave) (p<0.001) and by contrast, non-carriers experienced a 3.1 increase from 7.6 (1st wave) to 10.7 (2nd wave) (p<0.001).

Cognitive score changes depending on the presence of APOE ε4 in (A) healthy control, (B) Alzheimer’s disease, (C) Other dementia. APOE, apolipoprotein E.
In the other dementia group, the interaction between APOE ε4 carriers and ε4 non-carriers was not significant (F=0.035, p=0.855, η2=0.003) and the main effect of time was not also significant (F=2.03, p=0.182, η2=0.156).
Sex and APOE ε4 on cognitive decline rate
For female sex group (n=195), the cognitive score changes between APOE ε4 carriers and non-carriers was significantly different (F=5.919, p=0.016, η2=0.007), and the main effect of time was also statistically significant (F=25.291, p<0.001, η2=0.029). The mean cognitive impairment value for APOE ε4 carriers increased by 3.4 from the 1st to 2nd wave (p<0.001). Non-carriers demonstrated a decline of 1.4 during the 7-year hiatus (p=0.036) (Figure 2).
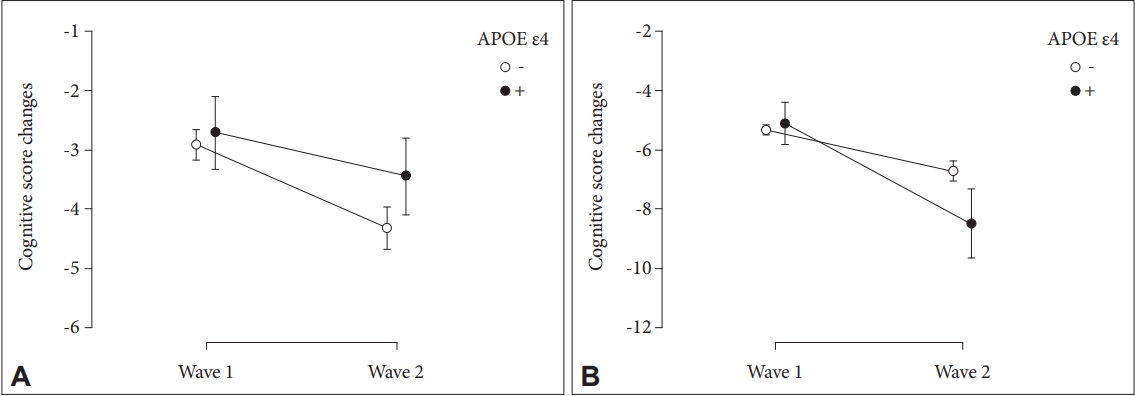
. Cognitive score changes depending on the presence of APOE ε4 in (A) male group and (B) female group. APOE, apolipoprotein E.
In the male sex group (n=162), the cognitive score changes between APOE ε4 carriers and non-carriers was not different (F=1.537, p=0.217, η2=0.002). The main effect of time was significant (F=5.103, p=0.025, η2=0.007). Familial dementia history, years of formal education, and illiteracy were set as covariates for both groups.
Education and APOE ε4 on cognitive decline rate
The population was grouped into no, low and high education groups based on academic completion. The subjects in the high-level group (n=13) completed secondary education and beyond. Individuals in the low-level group (n=119) finished primary education. Also known as “basic school,” primary education when these participants underwent school was approximately 3 years in length in the rural regions such as Yonchon. Those that did not finish primary education were included in the no education group (n=221).
For the no education group, the cognitive score changes between APOE ε4 carriers and non-carriers were significantly different (F=3.908, p=0.049, η2=0.004), and the main effect of time was also statistically significant (F=41.039, p<0.001, η2=0.041) after adjusting for familial dementia history. As shown in the Figure 3A, the mean cognitive impairment value for APOE ε4 carriers increased by 3.1 from the 1st to 2nd wave (p<0.001). Non-carriers demonstrated a lower decline of 1.6 during the 7-year hiatus (p=0.003).

Cognitive score changes depending on the presence of APOE ε4 in (A) no education group, (B) low education and (C) high education group. APOE, apolipoprotein E.
In the low education group, the cognitive score changes APOE ε4 carriers and non-carriers was not different (F=2.475, p=0.119, η2=0.006). The interaction was also insignificant in the higher education group (F=0.185, p=0.678, η2=0.003). The main effect of time was insignificant for both the low and high education groups.
DISCUSSION
Using a community-based study of the Korean elderly, we examined the role of the APOE ε4 allele to the incidence of dementia (i.e., AD and other dementia) and their interactions on cognitive function depending on factors of AD presence, sex, and education. In this study, the correlation between the onset age of dementia and the presence of the APOE ε4 allele was not statistically significant. However, longitudinally collected cognitive measurements demonstrated that the APOE ε4 was associated with steeper decline in AD groups. Cognitive decline was greater in the presence of the APOE ε4 allele (score change=7.4) than its absence (score change=3.1), and this interaction was significant only in the AD group (F=10.51, p=0.003). In the other dementia group, the interaction between APOE ε4 carriers and non-carriers was statistically insignificant. The influence of the APOE ε4 allele on dementia progression is supported as astrocytes and neurons expressing the variant reduce lipid-binding capacity, augment intracellular degeneration, and decrease in phospholipid and cholesterol secretion [26]. The association between the ε4 variant and accelerated longitudinal cognitive decline has also been shown in community studies of both the Caucasian and Asian populations [27,28].
From the aspect of relative risk of dementia (i.e., AD and other dementia), it was not statistically significant depending on the presence of the APOE ε4 allele in present study. This finding was consistent with existing results community studies in the African, Hispanic, and Asian demographic [29-33]. Previous investigations that reported the ε4 variant as a significant risk factor for AD were largely clinical studies conducted in institutions such as nursing homes, dementia research center, and hospitals [1,25,34]. This discrepancy in the significance of the APOE ε4 allele can be alluded to the distinct types of studies and suggest the possibility of selection bias through the variant’s role in accelerating cognitive decline. We observed that non-carriers of the ε4 variant experience slower dementia progression and thus regard their cognitive change as a normal process of aging. Carriers of the allele, however, may experience significantly accelerated dementia progression and recognize their cognitive decline as a medical concern. As a result, this difference in dementia progression may culminate in clinical studies encountering increased correlation between dementia incidence and the APOE ε4 allele.
This study showed APOE ε4 to be sex-dependent, accelerating cognitive decline by a factor of more than 2 times for female carriers. Analysis of the cognitive score changes demonstrated that APOE ε4 was significantly associated with sex when corrected for years of education, illiteracy, and familial dementia history. In the female group, cognitive decline was greater in APOE ε4 carriers (score change=3.4) than non-carriers (score change=1.4) and this interaction was statistically significant (F=5.919, p=0.016). By contrast, in the male group, however, interaction between APOE ε4 presence and cognitive score change was not statistically significant. This finding is supported by meta-analysis that reported greater cognitive decline in female carriers than their male counterparts [35]. The mechanism behind APOE ε4-AD risk in women is not clear until now and Dubal and Rogine [36] suggest the needs to explore the mechanisms of female vulnerability, such as whether gonadal hormones or sex chromosome mediate the APOE ε4 sex difference.
While the correlation between education duration and cognitive decline was statistically insignificant, the interaction between APOE ε4 and formal education experience was significant. Cognitive decline was greater in APOE ε4 carriers (score change=3.0) than non-carriers (score change=1.5) only in no education group and this interaction was statistically significant (F=3.903, p=0.05). In both the low and high education group, the difference between cognitive impairment change for APOE ε4 carrier and non-carrier was insignificant. Previous studies indicate that longer education duration confers greater protection from the APOE ε4 effects [12,37,38]. Makkar et al. [16] reported high school education was related to decreased cognitive impairment risk in Black APOE ε4 carriers but in Whites, related to a significant decrease in cognitive impairment risk in non-carriers.
We can consider the unique historical context of our subjects which provides a possible explanation for the divergence between our findings and previous reports. Our sample population received education between the 1920s and early 1940s, a period characterized by the Japanese Annexation of Korea (1910–1945). During this colonial period, Japanese replaced Korean as the medium of instruction, however, the Korean language remained readily spoken among the Korean population, fostering dual language usage among students receiving colonial education. Past studies have reported that active bilingualism is a is a significant proxy for cognitive reserve and delays onset of cognitive impairment [39-41]. We examined neutralized APOE ε4 effects among subjects that at least completed a primary education delivered by a foreign language. This strengthens evidence for the potency of early education and suggests that dual language learning in early childhood may promote protection against the APOE ε4 allele similar to the effects observed from active bilingualism on cognitive impairment. To our knowledge, this is the first study to suggest that dual language competency in early childhood can confer protection against APOE ε4.
Our sample population demonstrated an insignificant difference between high and low education durations, suggesting that impact of low-quality education on cognitive decline is independent from education years beyond completion of primary school. Previous studies suggested that education factors beyond years such as community factors may influence cognitive reserve and cognitive decline [42-44] and the relationship is curvilinear. Wilson et al. [45] reported that additional education does not contribute to further significant reductions in cognitive decline after a certain level of educational attainment and other studies have suggested that this may occur after completion of 8–9 years [46,47]. Further investigation may be needed regarding the quality of education and active bilingualism on cognitive reserve.
A limitation of our study is that APOE ε4 genotyping was not available for all of the participants, prompting the possibility that the frequency of the ε4 variant may not be an accurate representation of the study population. In this study, there was no significant correlation between the onset age dementia and the presence of APOE ε4 allele. This result may be caused by the selection bias in this study. Further study to represent whole community-based cohort would be needed.
However, our investigation has the importance to focus the association between dementia progression and the APOE ε4 allele through the drug naïve and community study. This study suggests that Clinicians examining patients with rapid declining dementia should consider the presence of APOE ε4 allele as well as the dementia type.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Jun-Young Lee, Soowon Park. Data curation: Alexander Han. Formal analysis: Alexander Han, Jun-Young Lee. Funding acquisition: Jun-Young Lee. Investigation: Jun-Young Lee, Soowon Park. Mehodology: Jun-Young Lee. Project administration: Jun-Young Lee. Resources: Jun-Young Lee. Supervision: Jun-Young Lee, Soowon Park. Validation: So Young Yoo, Alexander Han. Visualization: Alexander Han. Writing—orginal draft: Alexander Han, So Young Yoo. Writing—review & editing: Jun-Young Lee, Soowon Park.
Funding Statement
This study was supported by grants from the Korea Research Foundation Grant (KRF-2002-041-E00150) and the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A050079).

