A Single Baseline Amyloid Positron Emission Tomography Could Be Sufficient for Predicting Alzheimer’s Disease Conversion in Mild Cognitive Impairment
Article information
Abstract
Objective
Baseline amyloid burden in mild cognitive impairment (MCI) has been linked to conversion to Alzheimer’s disease (AD), but the comparison of baseline and longitudinal changes in amyloid burden for predicting AD remains unresolved. The objectives of this study aimed to compare the prognostic ability of baseline and longitudinal changes in amyloid burden in MCI patients.
Methods
Seventy-five individuals with MCI were recruited and examined annually by clinical interviews for a mean follow-up of 24 months (range, 11.6–42.0). [18F]Florbetaben positron emission tomography (PET) scans were performed. T1-weighted 3D volumes were acquired for co-registration, and to define regions of interest. We examined whether baseline and longitudinal amyloid burden changes can improve AD conversion by Cox proportional hazard model analysis and receiver operating characteristic (ROC) curve analysis.
Results
Cox proportional hazards model analysis showed that baseline amyloid burden was significantly associated with increased risk of conversion to AD (hazard ratio [HR]=10.0; 95% confidence interval [CI], 1.15–85.39; p=0.04), but longitudinal amyloid burden changes was not (HR=0.2; 95% CI, 0.02–1.18; p=0.07). When predicting AD, longitudinal amyloid burden changes had better ROC accuracy of 65.2% (95% CI, 48.4–82.0) than baseline amyloid burden of 59.6% (95% CI, 40.3–79.0), without statistical significance in pairwise comparison.
Conclusion
A single baseline amyloid PET could be sufficient in the prediction of AD conversion in MCI.
INTRODUCTION
Mild cognitive impairment (MCI) is the intermediate stage between normal aging and dementia. Individuals with MCI shows annual conversion rates to probable Alzheimer’s disease (AD) of approximately 10%–15% per year, while the rate in normal elderly people is 1%–2% [1]. It has been reported that MCI with high amyloid retention had 67%–82% conversion rates to AD during 2–3 years of follow-up [2-4]. However, these studies evaluated only baseline amyloid burden which could not assess serial amyloid changes during the clinical follow-up period. Subsequently, a few follow-up studies have evaluated both serial amyloid burden changes and MCI conversion to AD [5-9]. Although their follow-up duration was a maximum of 5.7 years, the included baseline MCI individuals were less than 50 in number (minimum was 10). Moreover, they did not compare amyloid burden interval changes with baseline amyloid burden for the prediction of MCI conversion to AD.
Therefore, our study aimed to compare baseline amyloid burden and longitudinal amyloid burden changes in the prediction of MCI to AD.
METHODS
Participants
Seventy-five individuals with MCI were recruited from the community and from the Dementia Clinic, Chosun University Hospital, Gwangju, South Korea from April 2017 to January 2021. All subjects were fully informed about study participation, and written informed consent was obtained from all participants. All participants were examined by clinical interview, which included an assessment of the Clinical Dementia Rating (CDR) [10]. The participants received a CDR score of 0.5 and met Petersen’s criteria [11], which includes: 1) memory complaint corroborated by an informant, 2) objective cognitive impairment of age, education and sex, 3) essentially preserved general cognitive function, 4) largely intact functional activities, and 5) not demented. For criterion 2), a z-score performance for at least one of the attention, memory, language, visuospatial function, and frontal/executive cognitive function tests included in the Seoul Neuropsychological Screening Battery (SNSB) was below -1.5 according to the respective age-, education- and sex-specific norms. Individuals with MCI were classified as either amnestic MCI (aMCI) or nonamnestic MCI (naMCI) by including neuropsychological memory test impairments in addition to MCI diagnostic criteria.
The exclusion criteria included any current serious medical, psychiatric, or neurological disorder affecting the patient’s cognitive function, evidence of focal brain lesions on MRI including multiple lacunes and white matter hyperintensities (WMH) of grade 2 or more on the Fazekas scale [12]; presence of severe behavioral or communication difficulties; or a current use of psychoactive medication.
The Institutional Review Board of Chosun University Hospital approved the study protocol (CHOSUN 2016-12-011-003).
Clinical and neuropsychological assessments
All participants were examined annually by a clinical interview. Their medical history, including stroke or family history of dementia, was also assessed. Clinical diagnoses including CDR scores, were made after reviewing all available information in consensus case conferences (authors of IHC, AC, JYC, JMH, HK). MCI converters were defined when the follow-up diagnosis was AD following National Institute on Aging–Alzheimer’s Association diagnostic criteria [13]. MCI non-converters were defined when the follow-up diagnosis was not AD including reversion to cognitively normal and MCI again. A comprehensive neuropsychological assessment was also performed annually using the SNSB II [14], which covers five cognitive domains. The attention domain was assessed using a forward and backward digit span test. The language domain was assessed using a shortened form of the Korean version of the Boston Naming Test (BNT, 15-item version, Form A). The visuospatial domain was assessed using the copying test from the Rey Complex Figure Test (RCFT). The memory domain was assessed by six measures: the Seoul Verbal Learning Test (SVLT) immediate recall (SVLTirl), SVLT 20-minute delayed recall (SVLTdrl), SVLT yes-no recognition (SVLTrcg), RCFT immediate recall (RCFTirl), RCFT 20-minute delayed recall (RCFTdrl), and RCFT yes-no recognition (RCFTrcg). The frontal/executive domain was assessed by category fluency tests (animal and supermarket lists), Stroop test (Stroop_W, word reading; Stroop_CW, color naming in the color-word incongruent condition), and Trail Making Tests A and B. Global cognition was assessed using the Mini-Mental State Examination.
Image acquisition
T1-weighted 3D volumes were acquired (3T, Siemens AVANTO) for co-registration with positron emission tomography (PET) and to define the region of interest (ROI). Additionally, fluid-attenuated inversion recovery images were obtained for WMH readings.
PET scans were performed using a PET/CT scanner (Discovery ST PET/CT, GE) with a field of view of 250 mm, providing slices of 3.3 mm thickness and 256×256 matrix size. The brain 3D acquisition mode was used. Images were reconstructed from the data with ordered-subset expectation maximum iterative reconstruction algorithm (4 iterations, 32 subsets). After [18F]Florbetaben injection, subjects were allowed to wait for 90 min, and 47-slice images were acquired over 20 min. The injected dose was 300 MBq for every subject.
Image analysis
[18F]Florbetaben images were co-registered and re-sliced into individual T1 reference images using Statistical Parametric Mapping (SPM) 12 (Wellcome Trust Centre for Neuroimaging) software based on MATLAB (The MathWorks, Inc., Natick, MA, USA). All T1 reference images were segmented into the grey matter (GM) and WMH tissue classes using the unified segmentation algorithm of SPM12. The resultant probabilistic GM density map for each participant had a threshold of 0.5 applied to it, and a binary GM mask was thus created (0, no tissue; 1, tissue with a >50% probability of belonging to GM). The inverse nonlinear transformation parameter file from the segmentation algorithm of SPM12 was used to warp a simplified digital probabilistic atlas using automated anatomical labeling [15], consisting of 120 cortical and subcortical regions, into each participant’s native T1 space. These atlases were multiplied by the corresponding binary GM mask, which generated a GM-specific digital atlas for each participant. Raw, co-registered, and re-sliced PET and MRI data for each participant were sampled using the same individual digital atlases created previously. The mean regional standardized uptake value ratios (SUVRs) were measured for each atlas region using this method. Regional amyloid binding ratios were acquired by dividing each atlas region by the respective mean cerebellar GM values. The mean cortical Aβ burden was expressed as the average SUVR of the area-weighted mean of the frontal, medial temporal, lateral temporal, lateral parietal, posterior cingulate-precuneus, basal ganglia, and occipital regions [16], which were also defined as ROIs for the exploratory first step partial correlation analyses.
Statistical analysis
Statistical analyses were performed using SPSS Version 22.0 (Statistical Package for the Social Sciences; IBM Co., Armonk, NY, USA) and R statistical software version 3.3.3 (R Project for Statistical Computing, Vienna, Austria) within RStudio. Demographic and clinical data from the two groups (converter and non-converter) were compared using an unpaired t-test, while the χ2 test was applied to compare proportions and categorical data. We assessed the effect of each biomarker variable with baseline mean cortical amyloid SUVRs and longitudinal mean cortical amyloid SUVR changes on time to a diagnosis of AD among subjects diagnosed with MCI at baseline using the Cox proportional hazards models that included age, sex, education, and apolipoprotein (APOE) e4 allele as adjustment covariates. The baseline visit was considered to be time zero. The longitudinal amyloid burden changes were defined as the last follow-up mean cortical SUVR minus the baseline mean cortical SUVR.
The AD prediction classification accuracy of the resulting models was calculated using receiver operating characteristic (ROC) curve analysis. The resulting models had baseline amyloid burden and longitudinal amyloid burden changes for MCI conversion prediction. Pairwise ROC curve comparisons for AD prediction models between baseline amyloid burden and longitudinal amyloid burden changes were calculated using the DeLong method [17]. All ROC analyses were performed also for two subgroups of individuals with a follow-up duration of less than 24 months and participants with a follow-up duration of 24 months or more.
RESULTS
Participants
Seventy-five MCI subjects were examined using annual follow-up diagnosis. Of the 75 MCI participants, 17 (22.7% total, or an annual rate of 11.4%) converted to AD. The mean followup duration was 24 months (standard deviation [SD]=9.3; range, 11.6–42.0). Of the 75 MCI participants, 63 underwent second or/and third amyloid PET and MRI for follow-up. Clinical evaluations were performed within 1 month of amyloid PET and MRI. Of 63 MCI patients, 11 converted to AD dementia and 22 remained MCI at the second amyloid PET, and 6 converted to AD dementia and 24 remained MCI at the third amyloid PET.
Table 1 summarizes the demographic information and clinical manifestations for baseline measurements for MCI converter and non-converter groups. MCI converters did not show statistically significant differences in terms of age, education, mean cortical amyloid burden, CDR, Subjective Memory Complaints, Geriatric Depression Scale, and APOE ε4 allele ratios, compared to MCI non-converters. However, MCI converters had significantly lower female frequency than MCI non-converters (29.4% vs. 56.9%, p=0.046). Furthermore, MCI converters had significantly lower z-scores than MCI non-converters in the BNT (p=0.003), SVLTdrl (p=0.023), RCFTdrl (p=0.006), and Stroop Color Word test (p=0.007).
Diagnostic changes during follow up
Table 2 summarizes the clinical diagnostic changes in baseline MCI during follow-up. Of these, 28 (37.3%) had stable aMCI diagnoses (from aMCI to aMCI), nine (12%) had stable naMCI (from naMCI to naMCI), 15 (20.0%) interchanged between aMCI and naMCI, 12 aMCI patients converted to AD (16.0%), five naMCI converted to AD (6.7%), five aMCI reverted to CN (6.7%) and one naMCI reverted to CN (1.3%). Patients in the two aMCI and naMCI to AD conversion groups were defined as MCI converters (n=17), and the other six groups were categorized as MCI non-converters (n=58).
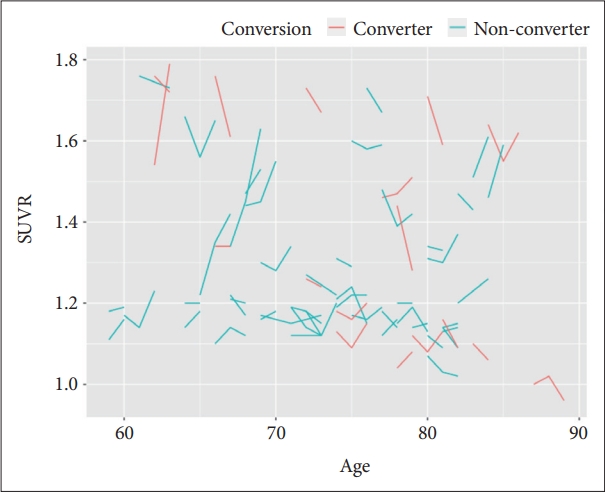
Longitudinal amyloid burden changes during follow up
Figure 1 demonstrates longitudinal amyloid burden SUVR changes in follow-up time, which were classified as MCI converters (n=17) and MCI non-converters (n=46). For first and second follow-up subjects (n=33 and n=30), follow-up duration means were 16.3 and 32.3 months (SD, 6.7 and 4.8). Until last image follow-up with clinical diagnosis, mean amyloid burden change (follow-up SUVR–baseline SUVR) was -0.011 (SD, 0.16; range, -1.10–0.29).
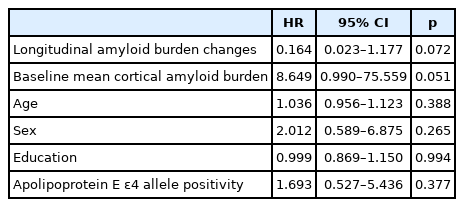
Cox proportional hazards models with conversion to Alzheimer’s disease as the outcome measures
The age, sex, education, and APOE ε4 allele-adjusted HR for baseline mean cortical amyloid burden was 10.1 (p=0.04; 95% confidence interval [CI], 1.15–85.39) (Table 3). The age, sex, education, APOE ε4 allele, and baseline mean cortical amyloid burden adjusted hazard ratio (HR) for longitudinal amyloid burden changes was 0.2 (p=0.07; 95% CI, 0.02–1.18) (Table 4).

Baseline factors of Alzheimer’s disease prediction in mild cognitive impairment analyzed by Cox proportional hazards model
Receiver operating characteristic curve analysis of mild cognitive impairment conversion to Alzheimer’s disease prediction models
Mild cognitive impairment conversion to Alzheimer’s disease prediction models based on baseline amyloid burden
The MCI conversion to AD prediction model for the baseline amyloid burden showed that the overall ROC accuracy was 59.6% (95% CI, 40.3–79.0). For individuals with a follow-up duration of less than 24 months, the overall ROC accuracy was 71.1% (95% CI, 47.3–95.0). For participants with a follow-up duration of 24 months or more, the overall ROC accuracy was 58.3% (95% CI, 27.1–89.6) (Figure 2).
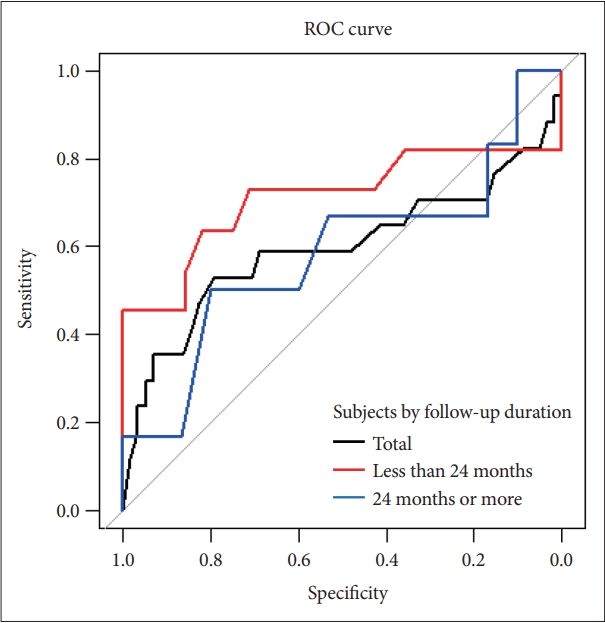
ROC curves for MCI conversion to Alzheimer’s disease prediction models by baseline mean cortical amyloid burden. ROC curves for total MCI individuals (black line), ROC curves for individuals with less than 24 months follow-up duration (red line), ROC curves for individuals with 24 months or more follow-up duration (blue line). ROC, receiver operating characteristic; MCI, mild cognitive impairment.
Mild cognitive impairment conversion to Alzheimer’s disease prediction models by longitudinal amyloid burden changes
The MCI conversion to AD prediction model for the longitudinal amyloid burden changes showed that the overall ROC accuracy was 65.2% (95% CI, 48.4–82.0). For individuals with a follow-up duration of less than 24 months, the overall ROC accuracy was 75.5% (95% CI, 53.9–97.1). For participants with a follow-up duration of 24 months or more, the overall ROC accuracy was 52.4% (95% CI, 30.5–74.3) (Figure 3).

ROC curves for the MCI conversion to Alzheimer’s disease prediction models by longitudinal amyloid burden changes. ROC curves for total MCI individuals (black line), ROC curves for individuals with less than 24 months follow-up duration (red line), ROC curves for individuals with 24 months or more follow-up duration (blue line). ROC, receiver operating characteristic; MCI, mild cognitive impairment.
Comparisons of the mild cognitive impairment conversion to Alzheimer’s disease prediction between baseline amyloid burden and longitudinal amyloid burden changes
Pairwise ROC curve comparisons for AD prediction models between baseline amyloid burden and longitudinal amyloid burden changes showed no significant area under the curve difference for all MCI participants (p=0.663). For participants with a follow-up duration of fewer than 24 months (n=29) and participants with a follow-up duration ≥24 months (n=33), no significant differences were found (p= 0.782, p=0.742).
DISCUSSION
In the present study, we showed that an MCI conversion to AD prediction model based on the baseline amyloid burden had a 59.6% classification accuracy and that the longitudinal amyloid burden changes had a 65.2% classification accuracy.
In our study, the AD progression rate of amyloid-positive individuals with MCI was lower than that reported previously. Previous studies calculating the baseline amyloid burden have reported that 67%–82% of amyloid-positive participants with MCI progressed to AD in about 32 months [2-4,7,8]. However, the AD progression rate in the present study was 44%, although the mean follow-up duration was 24 months. This difference might be because we recruited individuals with MCI in the community, while previous studies were conducted in hospitals or private clinics. Of note, the present study had a baseline amyloid burden positive rate of 24% of total MCI subjects, compared with rates of 55%–69% found in hospital or clinic-based studies. Another reason might be that our study showed no significant amyloid SUVR difference at baseline between MCI converters and MCI non-converters, in contrast to previous studies reporting that MCI converters had significantly higher amyloid SUVR at baseline than MCI non-converters [2,7,8]. In addition, follow-up diagnostic changes in the present study showed that 76% of MCI patients had a stable diagnosis 2 or 3 years later, although 20% of them had minor diagnostic interchanges between aMCI and naMCI, and 8% MCI reverted back to cognitively normal. These diagnostic changes are in line with the community vs. clinic comparison MCI study [18]. Therefore, our study results could represent an AD progression model in the community of individuals with MCI.
The findings of the Cox proportional hazard model analysis showed that the HR for baseline mean cortical amyloid burden was 10.1 with statistical significance, the longitudinal amyloid burden changes was not significant. Pairwise comparisons of ROC curves showed that the longitudinal amyloid change model had a higher accuracy than the baseline amyloid model, in which statistical significance was not found. The different results between two analyses might be in that Cox proportional hazard model analysis adjusted age, sex, education, and APOE ε4 allele, but ROC curve analysis did not.
We believe that this might be the first study to demonstrate the ROC curve comparisons between the baseline amyloid model and longitudinal amyloid change model for MCI conversion to AD. Some studies have performed annual amyloid PET and diagnostic evaluation for the prediction of MCI conversion to AD [6,7,9,19,20]. However, they included a relatively small sample of 10–48 individuals with MCI, and did not apply the longitudinal amyloid burden change to assess the prediction of MCI conversion to AD. One study reported that the amyloid burden increase was 7%–62% at 5 years, with a mean of 26% (annual 5%), although some individuals showed a slight decrease [6]. Other large sample multicenter studies focused on longitudinal amyloid accumulation in cognitively normal or subtle cognitive decline individuals rather than MCI conversion to AD [21-24]. They demonstrated that the increase in beta-amyloid deposition is faster in the predementia stage, but not at a constant rate across the clinical stages of the AD spectrum. These variations in the serial amyloid burden change are in line with our findings of no significant difference between the baseline and the longitudinal amyloid burden change for the prediction of MCI conversion to AD.
Our study had several limitations. Firstly, we had a relatively short follow-up duration of a mean of 24 months and follow-up times variations between subjects, although one longitudinal follow-up study reported that MCI patients were diagnosed with AD on average at the 3-year clinical follow-up visit [6]. Thus, our results need to be supported with 3- or 5-year follow-up to confirm MCI conversion to AD prediction with serial amyloid changes. Second, our study included both aMCI and naMCI, which might have different progression rates. Further, in our observations, we only included participants with MCI and amyloid PET. However, future studies need to include more individuals across the categories of cognitively normal, MCI, and mild AD dementia, as well as tau PET.
Nevertheless, the results of the present study suggest that a single baseline amyloid burden could be sufficient in the prediction of AD conversion in MCI.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
IL Han Choo, a contributing editor of the Psychiatry Investigation, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions
Conceptualization: IL Han Choo. Data curation: all authors. Formal analysis: IL Han Choo. Funding acquisition: all authors. Project administration: IL Han Choo, Hoowon Kim. Visualization: Yu Yong Choi. Writing—original draft: IL Han Choo. Writing—review & editing: IL Han Choo.
Funding Statement
This study was funded by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. 2016M3A9E9941914).