Risk of Mental Illnesses in Patients With Hypopituitarism: A Nationwide Population-Based Cohort Study
Article information
Abstract
Objective
The associations of mental illnesses and hypopituitarism have been reported. But, pituitary disorders are rare. The epidemiological studies have rarely addressed these associations between pituitary disorder and mental illnesses. Until now, no cohort study has been conducted to investigate the association.
Methods
We performed a nationwide, retrospective cohort study using the Taiwanese National Health Insurance Program dataset to analyze this relationship. In total, 1,194 patients diagnosed with hypopituitarism between 2000 and 2013 were identified. For the control group, 4,776 individuals without hypopituitarism and psychotic diseases were matched (1:4) according to age, sex, and index date. A Cox proportional hazards model was used to determine the adjusted hazard ratio (aHR).
Results
Patients with hypopituitarism had a significantly higher risk of incident depression and anxiety disorders than those without hypopituitarism. The aHRs of depressive and anxiety disorders were 2.98 and 1.67, respectively, for the hypopituitarism cohort. Furthermore, the risk of both hypopituitarism-associated depressive and anxiety disorders was significantly high in female subjects and subjects aged ≥18 years. A statistically significant increase was not observed in the risk of bipolar disorders, dementia, or schizophrenia in the hypopituitarism group compared with the control group.
Conclusion
Although psychiatric morbidities were uncommon for the hypopituitarism cohort, the risk of developing depressive and anxiety disorders was significantly higher in those with hypopituitarism than in those without hypopituitarism.
INTRODUCTION
Hypopituitarism is a disease characterized by the decrease or absence of one or several hormones secreted by the pituitary gland. Panhypopituitarism refers to a condition in which all of the adenohypophyseal hormones are absent in a patient, whereas partial hypopituitarism refers to a condition in which only one or few of the pituitary hormones are absent [1,2]. Hypopituitarism consists of a group of metabolic diseases with high rates of mortality and morbidity [3,4]. In Western countries, the annual incidence rate of hypopituitarism was reported to be 4.21 per 100,000 people according to a study in Spain [5]. In Taiwan, data on the incidence rate of hypopituitarism are lacking, but it is estimated to be low. Associated comorbidities of hypopituitarism include hypothyroidism, adrenal insufficiency, pituitary dwarfism, hypogonadism, diabetes insipidus, and psychiatric manifestations, which can run either an acute or insidious course [6-8], and the diagnosis is often delayed [9].
In 1949, Sheehan and Summers [10] described depressive symptoms as well as psychosis in their study on hypopituitarism involving 143 patients. Several studies have reported depressive symptoms in patients with hypopituitarism [11-15]. Another study that analyzed 46 patients with hypopituitarism and untreated growth hormone (GH) deficiency reported an increased rate of major depressive disorder [16], a finding that was replicated in a study on 33 female patients with hypopituitarism [17]. Furthermore, few case reports have suggested an association between psychotic symptoms and hypopituitarism [13-15,18].
The mechanisms leading to psychotic disorders in patients with hypopituitarism have been hypothesized to be a combination of hypothyroidism, hypoglycemia, and adrenal insufficiency [18], GH deficiency [16], increased cardiovascular risk, comorbidities, and a history of radiotherapy for pituitary tumors [17]. Furthermore, in addition to glucocorticoid use [19], deficiencies related to the thyroid gland, adrenal cortex, and sex hormone [20-24] are common in these patients, which could play a role in this disease. Thus, patients with hypopituitarism are expected to have an increased risk of psychiatric diseases.
However, the onset of neuropsychiatric manifestations in patients with hypopituitarism is uncommon; there are few reports of patients with hypopituitarism presenting with psychosis or depression. Yet, it is believed that the incidence of psychiatric disorders is reported to be low because of inadequate sample size for examining the risk. To date, the association between psychotic diseases and hypopituitarism remains unclear. In addition, hypopituitarism-related psychosis may be overlooked because of slow progression, indolent course, delayed/uncorrected diagnosis, and nonspecific psychiatric and physical symptoms [5].
No cohort study has been conducted to investigate the association between hypopituitarism and mental illnesses. Thus, the risk magnitude remains unestablished. Therefore, we investigated the incidence of diagnosed mental illnesses in patients with hypopituitarism compared with controls using a large, nationwide, and population-based database from Taiwan.
METHODS
Data source
We conducted a large nationwide, retrospective, cohort study using the National Health Insurance Research Database (NHIRD) obtained from the Taiwanese National Health Insurance (NHI) records. The NHI is a form of nationwide health insurance that covers 99.9% of the 23.74 million people living in Taiwan. The Longitudinal Health Insurance Database (LHID), a subset of the NHIRD, comprises detailed healthcare usage data from 2000 to 2013 of 1 million NHI enrollees who were randomly selected and proven to be representative of the Taiwanese population by the National Health Research Institutes (http://nhird.nhri.org.tw/date_01.html). The investigated diseases in the database are coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The personally identifiable numbers in the LHID are encrypted before releasing to researchers. The Institutional Review Board of China Medical University Hospital, Taiwan approved this study (CMUH-104-REC2-115).
Study subjects
Using the LHID, we selected 2,660 patients with suspected panhypopituitarism (ICD-9-CM: 253.2) or partial hypopituitarism (ICD-9-CM: 253.3 and 253.4) (Figure 1). The diagnosis date was set as the index date. Because the ICD-9 system does not specify the central (thalamus/pituitary) or primary (thyroidal/adrenal/gonadal) origin of hypothyroidism, adrenal insufficiency, hypogonadism, hyponatremia, and diabetes insipidus, in this study, patients with endocrine disturbance related to hypothyroidism (ICD-9-CM: 244.0, 244.3, 244.8, and 244.9 excluding iatrogenic etiology), adrenal insufficiency (ICD-9-CM: 255.41 and 255.42), hyponatremia (ICD-9-CM: 276.1), ovarian failure (ICD-9-CM: 256.2, 256.31, and 256.39), female infertility (ICD-9-CM: 628), amenorrhea (ICD-9-CM: 626), testicular hypofunction (ICD-9-CM: 257.2), and testicular dysfunctions (ICD-9-CM: 257.8 and 257.9) were excluded.
Furthermore, 61 patients with mental illnesses, including neurocognitive disorders (ICD-9-CM: 290, 290.0, 290.1, 290.11, 290.12, 290.13, 290.1, 290.2, 290.20, 290.21, 290.3, 290.8, 290.9, 294.1, 294.10, 294.11, 294.8, and 294.9), schizophrenic disorders (ICD-9-CM: 295, 295.0, 295.1, 295.2, 295.3, 295.4, 295.5, 295.6, 295.7, 295.8, and 295.9), delusional disorders (ICD-9-CM: 297, 297.0, 297.1, 297.2, 297.8, 297.9, and 293.81), bipolar disorders (ICD-9-CM: 296.0, 296.1, 296.4, 296.5, 296.6, 296.7, 296.8, 296.80, 296.81, 296.82, 296.89, 296.9, 296.90, and 290.99), depressive disorders (ICD-9-CM: 296.2, 296.3, and 300.4), and anxiety disorders (ICD-9-CM: 300.0, 300.00, 300.01, 300.02, 300.09, and 300.3), before the index date were excluded.
We identified 1,194 patients with panhypopituitarism or partial hypopituitarism during the 2000–2013 period as the hypopituitarism cohort (Figure 1). Then, we matched patients without a diagnosis of partial hypopituitarism or panhypopituitarism (ICD-9-CM: 253.2, 253.3, and 253.4) as in year 2000 for age, sex, and index year at a ratio of 1:4 as the control cohort.
Outcome measures
The primary outcome measured was the diagnosis date of mental illnesses. Mental illnesses, including neurocognitive disorders (ICD-9-CM: 290, 290.0, 290.1, 290.11, 290.12, 290.13, 290.1, 290.2, 290.20, 290.21, 290.3, 290.8, 290.9, 294.1, 294.10, 294.11, 294.8, and 294.9), schizophrenic disorders (ICD-9-CM: 295, 295.0, 295.1, 295.2, 295.3, 295.4, 295.5, 295.6, 295.7, 295.8, and 295.9), delusional disorders (ICD-9-CM: 297, 297.0, 297.1, 297.2, 297.8, 297.9, and 293.81), bipolar disorders (ICD-9-CM: 296.0, 296.1, 296.4, 296.5, 296.6, 296.7, 296.8, 296.80, 296.81, 296.82, 296.89, 296.9, 296.90, and 290.99), depressive disorders (ICD-9-CM: 296.2, 296.3, and 300.4), and anxiety disorders (ICD-9-CM: 300.0, 300.00, 300.01, 300.02, 300.09, and 300.3), were identified. All patients were followed from the index date until the date of the selected mental illnesses diagnosis, withdrawal from the NHI, death, or the end of 2013, whichever occurred first. In addition, we analyzed sociodemographic characteristics as potential confounders. The sociodemographic characteristics consisted of age (<18, 18–40, and ≥40 years), sex, occupation, and urbanization level of residence. Occupation was divided into three categories: white collar (those with long indoor working hours, such as institution workers, civil servants, and businesspeople), blue collar (those with long outdoor working hours, such as farmers, fishermen, and industrial laborers), and other (such as those retired, unemployed and with a low income). Urbanization level was divided into four levels based on an NHI report, ranging from the most urban area (Level 1) to the most rural area (Level 4).
Statistical analysis
We compared the sociodemographic differences between the hypopituitarism and control cohorts using a chi-square test for categorical variables or a Student’s t-test for continuous variables. Continuous data were expressed as mean (standard deviation). To calculate the diagnosis risk of mental illnesses in question, we used the Cox proportional hazard regression model. With the incidence rate (per 10,000 person-years) of the control cohort for reference, crude and adjusted hazard ratios (aHRs) were calculated for each category of psychiatric disorders studied. This would indicate the diagnosis risk of each of the psychiatric disorders for the study cohort compared with the control cohort. To investigate whether the association varies with age and sex, we conducted ageand sex-stratified analyses. A log-rank test was used to compare the cumulative incidences of common mental illnesses between the hypopituitarism and control cohorts. We also conducted additional analyses with unadjusted and adjusted Cox proportional hazards models to control for potential confounding factors (age, sex, occupation, and urbanization level of residence) to determine the hazard ratios (HRs) in patients with depressive or anxiety disorders compared with the age-, sex-, and calendar year-matched controls. We used the SAS statistical package (version 9.3; SAS Institute, Cary, NC, USA) to analyze the data. A p value of <0.05 was considered statistically significant.
RESULTS
Baseline characteristics of the study cohort
We identified 1,194 patients with partial hypopituitarism or panhypopituitarism for the hypopituitarism cohort and 4,776 age-, sex-, occupation-, and urbanization level-matched patients without the aforementioned diagnoses for the control cohort, the diagnosis duration being from 2000 to 2013 (Table 1). The mean age of the participants was approximately 22 years, and the predominant sex was female (72.1%). Subjects with a white-collar occupation were more than those with a blue-collar occupation (68.2% vs. 22.6%). In addition, 35.7% of the participants lived in the most urbanized areas and 31.4% lived in the second most urbanized areas. The mean follow-up durations were 7.12 years for dementia, 7.11 years for delusional disorders and schizophrenic disorders, and 6.92 years for depressive disorders, bipolar disorders, and anxiety disorders for the hypopituitarism cohort. For the control cohort, follow-up durations were 6.93 years for dementia, delusional disorders, and schizophrenic disorders and 6.83 years for depressive disorders, bipolar disorders, and anxiety disorders.
Risk of developing mental illnesses
The incidence rates of all mental illnesses except for delusional disorders were higher in the hypopituitarism cohort than in the control cohort (Table 2). Compared with the control cohort, the hypopituitarism cohort had a significantly higher risk of depressive (aHR=2.98, 95% confidence interval [CI]=1.81–4.91) and anxiety (aHR=1.67, 95% CI=1.17–2.37) disorders. Moreover, the hypopituitarism cohort had a greater risk of dementia (aHR=3.18, 95% CI=0.85–11.9), schizophrenia (aHR=2.66, 95% CI=0.75–9.52), and bipolar disorders (aHR=1.79, 95% CI=0.77–4.15) compared with the control cohort; however, the risk was nonsignificant.

Incidence rates and HRs of dementia, schizophrenia disorders, depressive disorders, bipolar disorders, and anxiety disorders between the hypopituitarism cohort and the comparison cohort
As shown in Table 3, patients in the hypopituitarism cohort were classified into subgroups on the basis of age and sex. The HR of depressive disorders was higher in older patients with hypopituitarism (≥18 years; aHR=3.81, 95% CI=2.20–6.59) than in the younger patients (<18 years; aHR=1.20, 95% CI=0.33–4.37) compared with the patients in the control cohort. Similar results were obtained for the age-specific HR of anxiety disorders in patients with hypopituitarism (≥18 years: aHR=1.77, 95% CI=1.20–2.61; <18 years: aHR=1.28, 95% CI=0.55–3.00). Thus, the older patients had a significantly increased risk of hypopituitarism compared with the younger ones. Moreover, the sex-specific HR for depressive disorders was higher in female patients with hypopituitarism (aHR=4.13, 95% CI=2.41–7.09) than in male patients (aHR=0.40, 95% CI=0.05–3.15). Similar findings were observed for the sexspecific HR for anxiety disorders in patients with hypopituitarism (female patients: aHR=1.88, 95% CI=1.29–2.74; male patients: aHR=0.80, 95% CI=0.27–2.34).

Sex- and age-specific incidence rates of depressive disorders and anxiety disorders in subjects with and without partial and panhypopituitarism and Cox model estimated HRs for patients with partial and panhypopituitarism
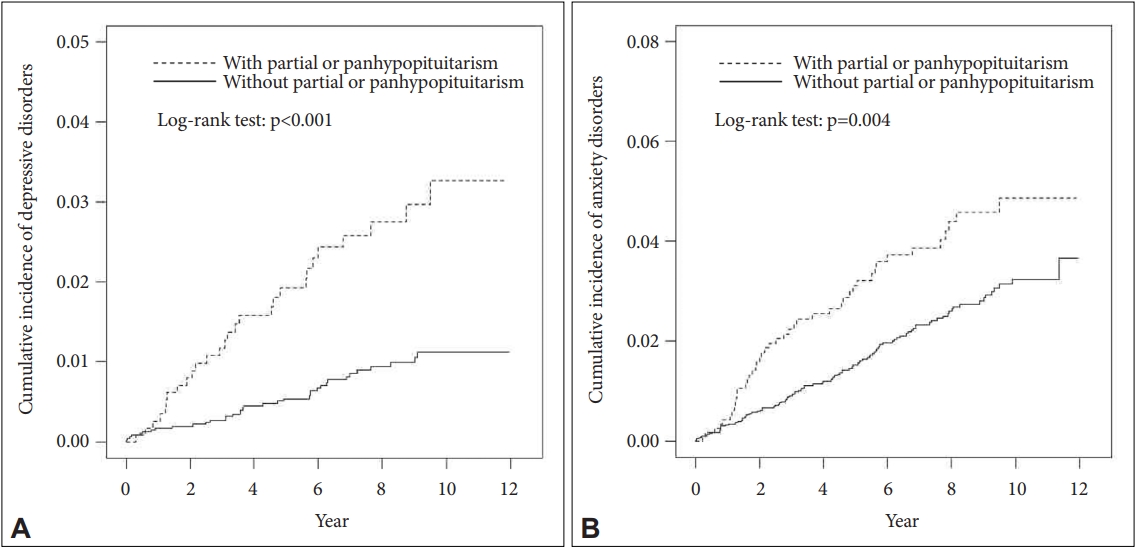
The cumulative incidence of depressive and anxiety disorders in patients with hypopituitarism with relation to time of follow-up is shown in Figure 2. The cumulative incidence, as calculated using the log-rank test, was higher in patients with hypopituitarism compared with those in the control cohort for patients with depressive or anxiety disorders throughout the study.
Confounding factors for the development of depressive and anxiety disorders
As shown in Table 4, to analyze the contribution of confounding factors in the pathogenesis of mental illness, the HRs of the risk of developing depressive and anxiety disorders throughout the study period were calculated for each of the sociodemographic divisions analyzed.
The risk of developing depressive disorders was higher in patients aged ≥18 years than in patients aged <18 years (≥18 years; aHR=4.74, 95% CI=2.26–9.94). The risk of depressive disorders was higher in female patients with hypopituitarism compared with male patients in the crude analysis (female; crude HR=2.14, 95% CI=1.12–4.09) and not in the adjusted analysis. Other demographic characteristics, namely occupation and urbanization, did not have a statistically significant association with depressive disorders.
In the crude analysis, those aged ≥18 years, females, whitecollar workers, blue-collar workers, and those residing in rural areas were shown to at a higher risk of developing anxiety disorders. However, these findings were not supported by the adjusted analysis. In the adjusted analysis, only having an age of ≥18 years (aHR=4.79, 95% CI=2.94–7.81) and being a bluecollar worker (aHR=2.90, 95% CI=1.14–7.39) were risk factors for the development of anxiety disorders throughout the study.
DISCUSSION
To our knowledge, this is the first large, nationwide, population-based, retrospective cohort study to evaluate the association between hypopituitarism and the risk of mental illnesses. We found a significantly increased risk of depressive and anxiety disorders in the hypopituitarism cohort compared with the control cohort. Furthermore, this study is the first to show that the risk of both depressive and anxiety disorders were significantly high in female subjects and subjects aged ≥18. However, statistically significant increase in the risk of developing bipolar disorders, dementia, or schizophrenia was not observed in patients with hypopituitarism.
One cross-sectional study and another case-control study investigated the correlation between hypopituitarism and the risks of depressive disorders [16,17]. The cross-sectional study in Sweden reported a 3.5-fold higher incidence of depression in 33 women with hypopituitarism and GH deficiency who were operated on for pituitary tumor; however, that study was based solely on self-rating questionnaires without additional verification of diagnoses [17]. Another case-control study that enrolled 41 adult patients with idiopathic GH deficiency and GH deficiency secondary to organic pituitary disease reported a 3.25-fold and 1.33-fold increase in the development of hypopituitarism-associated major depressive disorder and dysthymia, respectively, on the basis of a structured clinical interview [16]. Compared with previous cross-sectional and case-control studies, our longitudinal cohort study with a larger sample size revealed a similar but lower HR than that reported in these two studies [16,17]. Our data indicated that sex and age had significant effects on the risk of hypopituitarism. In terms of age, adults with hypopituitarism aged ≥18 years were found to have a significantly higher incidence of depressive disorders compared with the general population. This finding was not observed for children aged <18 years with hypopituitarism. Our study selected both adult and children with hypopituitarism; in contrast, in earlier two studies, only adult patients treated for hypopituitarism were included. This dissimilar study design may explain the differences in results; the overall HR of hypopituitarism increased from 3.07 to 3.77 when the analysis was restricted to adults.
In addition, regarding sex, the risk of depressive disorder for female patients was significantly higher in the hypopituitarism cohort than in the control cohort. This finding was not noted for male patients, indicating that sex played a role in hypopituitarism-related depressive disorders. Moreover, the female-to-male preponderances for depressive disorders in the hypopituitarism and control groups were 11.9:1 and 1.19:1, respectively (hypopituitarism cohort, 47.1 vs. 3.94 per 10,000 person-years; control cohort, 11.6 vs. 9.78 person-years). Thus, the risk of developing depressive disorders is relatively higher for female patients with hypopituitarism compared with male patients. Earlier studies on hypopituitarism have not compared male and female groups. We revealed that female patients with hypopituitarism had a 10-fold higher risk of developing depressive disorders than male patients. To our knowledge, this is the first study to present this finding. However, this is not surprising, because sex differences in patients with depressive disorders and pituitary gland complications have been reported in numerous studies. For example, sex differences in prevalence rates, risk factors, symptomatology, or disease course in patients with depressive disorders were identified [25]. Moreover, sex differences have also been studied regarding the pituitary size [26] and hypothalamic-pituitary-gonadal activation to stress [27]. However, the exact reasons for the sex difference in the incidence of hypopituitarism-related depressive disorders remain unclear. Further research is required on depression and pituitary-related hormones.
Similar observations on hypopituitarism-related depressive disorders were noted regarding hypopituitarism-related anxiety disorders. Individuals with hypopituitarism showed a significantly increased risk of anxiety disorders compared with the general population. Our findings were consistent with the earlier two studies, which reported that patients with hypopituitarism had higher scores regarding anxiety symptoms in Brief Anxiety Scale and Symptom Check List-90, respectively [16,17]. In addition, the age-specific HRs of anxiety disorders suggested that female sex and age of ≥18 years are risk factors for anxiety disorders in patients with hypopituitarism.
Furthermore, we found that our hypopituitarism cohort had higher incidences of dementia and schizophrenia compared with age- and sex-matched controls. However, the differences are nonsignificant. Although depressive and anxiety disorders occur in patients with hypopituitarism, patients with hypopituitarism rarely present with psychotic symptoms. Moreover, no systemic epidemiological study has been undertaken regarding schizophrenia and dementia in patients with hypopituitarism. Few case reports have noted that patients with hypopituitarism presented with psychotic symptoms such as hallucinations, paranoid delusions, irritability, cognitive decline, and even clinical misdiagnoses of schizophrenia [13,15,28-30]. Bülow et al. [17] reported that subjective self-rating by women with hypopituitarism indicated higher scores in terms of paranoid ideation and psychoticism scales. However, the study by Lynch et al. [16], which used a structured clinical interview, failed to find an association between hypopituitarism and ratings on the Comprehensive Psychopathological Rating Scale or its subscales. Accordingly, patients with hypopituitarism may vary in their vulnerability to psychosis manifestations.
The pituitary gland secretes several hormones, namely GH, prolactin, adrenocorticotropic hormone, thyroid-stimulating hormone, follicle-stimulating hormone, luteinizing hormone, melanocyte-stimulating hormone, antidiuretic hormone, and oxytocin, which regulate other essential endocrine glands, including the thyroid, pancreases, adrenal, and reproductive glands [31]. Overt hypopituitarism can affect a wide range of cognitive domains [32]. The clinical manifestations of hypopituitarism may resemble those of cognitive disturbances. In addition, patients with hypopituitarism-related GH deficiency, hypothyroidism, testosterone deficiency, or adrenal insufficiency may experience cognitive disturbances [33]. Therefore, it is reasonable to assume that patients with hypopituitarism have a higher incidence of dementia compared with those without. However, in our study, the incidence of dementia in patients with hypopituitarism was high, but nonsignificant in comparison with controls. To date, dementia has been rarely reported in patients with hypopituitarism. A case report described a 77-year-old man with hypopituitarism after a traumatic subdural hemorrhage presenting as long-term neuropsychiatric disorder, including cognitive impairment. However, these symptoms resolved almost completely after hormone replacement therapy [34]. Because cognitive impairment in patients with hypopituitarism is insidious, it may escape the attention of practitioners and be underdiagnosed [34]. In addition, a lack of knowledge about hypopituitarism, an uncommon disease for most clinical practitioners, may result in the underdiagnosis. However, these arguments must be considered speculative.
Depression or anxiety symptoms are sometimes reported in patients with hypopituitarism. Thus far, no study has reported bipolar disorder symptoms in patients with hypopituitarism. In our study, we found that hypopituitarism slightly increased the risk of bipolar disorder, but it did not achieve statistical significance. Therefore, hypopituitarism may not be correlated with a risk of bipolar disorder.
The main strengths of this study are the large nationwide sample and long follow-up period (13 years). The long followup period allowed for a comprehensive longitudinal view of the course of this disease, which has an extremely low incidence rate. The large sample size made it possible to evaluate the risk of mental illnesses in patients with hypopituitarism compared with the general population. However, this registry-based study has several limitations. The database records did not encompass essential confounding factors, including treatment accessibility, treatment adherence, and the severity of endocrine abnormalities at baseline and after treatment. The reported incidences of mental illnesses in our study were also registry-based. The diagnoses were based on interviews, but lacked the uniform questionnaires or structured interviews applied by previous investigators. In hypopituitarism diagnoses, much of the clinical information and confounders were lacking with regard to the diagnoses of mental illnesses. Furthermore, a confirmatory evaluation on mental illnesses using uniformed questionnaires was lacking.
Although the incidence of hypopituitarism is markedly low, 1,194 patients with hypopituitarism over a 13-year period were identified in this nationwide population-based study. Patients with hypopituitarism have higher incidences of depression and anxiety, which are particularly common in females and people aged ≥18 years. On the basis of these findings, we speculate that a small subgroup of females with hypopituitarism is susceptible to developing depression and anxiety. However, the causes of depression and anxiety remain unclear. Functional and metabolic neuroimaging studies combined with neuroendocrine assessments might elucidate the development of depression and anxiety in patients with hypopituitarism.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: I-Hua Wei, Chih-Chia Huang. Data curation: ChihChia Huang. Formal analysis: Chih-Chia Huang. Funding acquisition: I-Hua Wei, Chih-Chia Huang. Investigation: I-Hua Wei, Chih-Chia Huang. Methodology: I-Hua Wei, Chih-Chia Huang. Project administration: I-Hua Wei, Chih-Chia Huang. Resources: I-Hua Wei, Chih-Chia Huang. Software: Chih-Chia Huang. Supervision: Chih-Chia Huang. Validation: I-Hua Wei, Chih-Chia Huang. Visualization: I-Hua Wei, Chih-Chia Huang. Writing—original draft: I-Hua Wei, Chih-Chia Huang. Writing—review & editing: Chih-Chia Huang.
Funding Statement
This study was supported by the Ministry of Science and Technology, Taiwan (MOST 109-2314-B-039-040) and the China Medical University Hospital, Taiwan (DMR-110-127).