Age-Limited Effects of Low-Frequency Transcutaneous Electric Nerve Stimulation on Insomnia: A 4-Week Multi-Center, Randomized Controlled Study
Article information
Abstract
Objective
Insomnia disorder is a common condition with considerable harmful effects on health. We investigated the therapeutic efficacy and safety of low-frequency transcutaneous electric nerve stimulation (LF-TENS) as an alternative treatment option for insomnia disorder.
Methods
A 4-week, multi-center, randomized controlled study was conducted. A total of 160 individuals aged 40 to 80 years with insomnia disorder were included and randomized to the experimental group receiving active device (n=81) or control group receiving sham device (n=79). Both groups used the device for four weeks, more than five days a week. The participants also completed pre- and post-intervention assessment with questionnaires, sleep diaries, wrist actigraphy, and blood tests.
Results
There was no significant between-group difference in the changes of mood and sleep parameters and blood test results among the two study groups. Meanwhile, in the exploratory sub-group analysis of patients aged over 60 years, the experimental group showed better improvement after intervention in the change of Pittsburgh Sleep Quality Index (PSQI) score (-2.63±3.25 vs. -1.20±2.28, p=0.039; Cohen’s d=0.99 vs. 0.45) and blood cortisol level (-1.65±3.37 μg/dL vs. -0.16±3.49 μg/dL, p=0.007; Cohen’s d=0.56 vs. 0.05). In addition, no serious adverse reaction occurred during the study period in both groups.
Conclusion
The effect of LF-TENS was limited to older patients aged over 60 years, which might be related to the modulation of hypothalamic-pituitary-adrenal axis activity.
INTRODUCTION
Insomnia disorder is the most common sleep disorder characterized by dissatisfaction with sleep quality or quantity that causes considerable daytime functional impairment. Its prevalence has been estimated ranging from 10 to 33 percent in adults according to the reports of various studies conducted worldwide [1-4]. Moreover, a longitudinal study revealed that 46% of insomniacs complained of persistent insomnia symptoms even after three years, suggestive of chronicity of the disease [5]. Insomnia disorder is also associated with physical and mental health problems such as cardiovascular diseases [6], depressive disorder [7], and cognitive impairment [8].
The currently established treatment for insomnia disorder is restricted to short-term pharmacotherapy and cognitive behavior therapy (CBT), along with modification of precipitating and perpetuating factors for insomnia [9]. However, these conventional therapies have some limitations. The most widely used medications for insomnia including benzodiazepine receptor agonists have potential risk of side effects, such as dependency, tolerance [10] and cognitive decline [11]. CBT is costly and time-consuming because regular in-person encounters with therapists and good adherence of patients to the treatment sessions are required. Furthermore, over 40% of patients could not achieve remission with combined pharmacotherapy and CBT for six months [12]. To overcome these shortcomings of conventional therapies and to achieve better treatment outcomes, clinicians have tried to find an alternative therapeutic approach for insomnia.
Cranial electrotherapy stimulation (CES) is an electrical neurostimulation technique that delivers a pulsed, alternating low-intensity electric current across the head via electrodes on the earlobes or scalp. It was first introduced as a novel therapeutic modality for insomnia in the 1970s and since then, a couple of studies have indicated that CES could alleviate insomnia symptoms and improve sleep quality [13-15]. Meanwhile, some other researches have failed to confirm the therapeutic effects of CES on insomnia [16,17] and a recent systematic review also revealed inconclusive results regarding the efficacy of CES for insomnia treatment [18].
Transcutaneous electric nerve stimulation (TENS) is a different type of neurostimulation modality which applies electric current pulses by electrodes through peripheral skin surface other than the head. TENS was found to be effective for pain control in various pain conditions [19] and it is widely used for relieving neuromuscular pain with only minor adverse effects, including mild erythema and itching sensations underneath the electrodes [20]. In particular, low-frequency (<1,000 Hz) TENS (LF-TENS) is effective in local stimulation of the application site without cardiovascular side effects compared to highfrequency TENS (HF-TENS) [21]. Considering that chronic insomnia disorder is associated with inappropriate physiological hyperarousal [22] and that the possible analgesic mechanisms of LF-TENS include the activation of gamma-aminobutyric acid pathway [19], LF-TENS could be helpful for treating sleep disturbances. However, to our best knowledge, only few studies has examined the effects of LF-TENS on sleep [23,24] and no randomized controlled trial on this issue has been conducted.
To investigate safety and therapeutic effects of LF-TENS on insomnia, we previously performed an open trial with 40 patients suffering from chronic insomnia. Our data demonstrated that four weeks of intervention with LF-TENS can improve subjective sleep quality without intolerable or persistent adverse reactions [24]. However, the methodological shortcomings of this pilot study such as the absence of control group and objective sleep measurements may limit the interpretation of results. Hence, in the present study, we aimed to verify the safety and efficacy of LF-TENS application for the management of insomnia disorder, using a randomized double-blind and placebo-controlled parallel study design.
METHODS
Study population
The current study was conducted at two medical centers, Seoul National University Bundang Hospital and Asan Medical Center in Korea. People aged 40 to 80 years with chronic insomnia symptoms that lasted more than a year were recruited by advertisement in the local community. We included those who were diagnosed with insomnia disorder based on clinical interview at the first visit, using criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [25]. The exclusion criteria were as follows: 1) insomnia treatment history in the recent one year; 2) sleep apnea syndrome (apnea-hypopnea index ≥15 events/hr) or restless legs syndrome according to the diagnostic criteria of international restless legs syndrome study group (IRLSSG); [26] 3) major psychiatric disorders including mood disorders, anxiety disorders, dementia, and psychotic disorders; 4) medical illness such as cardiovascular diseases, malignancy, impaired renal or hepatic function, and lung diseases; 5) head trauma or seizure disorders; 6) infectious diseases; 7) diabetes mellitus on insulin therapy; and 8) pregnancy. All the participants were informed of the purpose and procedures of the study and they provided written consent at the first visit. All methods were performed in accordance with relevant guidelines and regulations. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (approval number E-1705/396-001) and the Korea Food and Drug Administration (approval number 786). The study protocol was retrospectively registered in the Clinical Research Information Service, Republic of Korea (registration number KCT0002705).
LF-TENS intervention
As a LF-TENS device, we used CR-9® medical device manufactured by Crown Medical, Inc. (Seoul, Korea). It was approved by the Korea Ministry of Food and Drug Safety and is currently commercially available for relieving muscular pain. The stimulator delivers an alternating current of <1 mA (peak output voltage ranges from 0.3 to 0.64 V) at a frequency of 400 Hz, with a pulse duration of 100 microseconds. Electric current is transmitted through three transcutaneous nickel plate electrodes attached to the back and neck area to stimulate both trapezius muscles. The sham device was created to look identical to the CR-9® device (Crown Medical, Inc.), but it has no electrical stimulation function.
Eligible participants were randomly assigned in a 1:1 ratio to either the experimental group receiving an active device, or control group receiving a sham device. The random assignment was performed via the stratified permuted block randomization method, using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and both patients and investigators were blind to the intervention condition. After assignment, patients were instructed to wear the device for an hour just before bedtime, more than five days weekly, for four weeks. Although the optimal dose and duration of LF-TENS application for insomnia treatment were not established, our preliminary study demonstrated the modest efficacy and safety of CR-9® medical device (Crown Medical, Inc.) for improving chronic insomnia, using the same protocol of device use [24]. A recent review has also indicated that sufficient therapeutic effects could be attained by daily use of electrical stimulation for more than four weeks, with a session duration of 20 to 60 minutes [27]. The patients were allowed to take a rescue medication (zolpidem 5 to 10 mg) if they experience the aggravation of insomnia symptoms for more than five consecutive days or reduction of total sleep time below three hours at least one day. For verifying the safety of the device use, we have checked the occurrence of any adverse event after two and four weeks of intervention. All the adverse reactions were recorded, using codes specified in the World Health Organization adverse reaction terminology (WHO-ART) version 92, updated by the Uppsala monitoring center (https://who-umc.org/vigibase/vigibase-services/who-art/).
Demographic and clinical characteristics
At the first visit, demographic and anthropometric data were gathered, including age, sex, body mass index (BMI), and medication use. Simultaneously, the presence of any medical illness, psychiatric disorders, or sleep disorders was assessed through detailed interview done by authors (H.J. Lee and H. Choi). And then all the individuals underwent an overnight polysomnography (PSG) at the sleep clinic of Seoul National University Bundang Hospital for screening out those with sleep apnea syndrome (apnea-hypopnea index ≥15 events/h). An EmblaTMN7000 device (Embla, Reykjavik, Iceland) was used for PSG. Sleep stages and respiratory events were scored by PSG technicians and sleep specialists every 30 seconds epoch, according to the American Academy of Sleep Medicine criteria [28].
Before and after treatment, participants were asked to complete self-report questionnaires concerning sleep quality, psychiatric symptoms, and pain. The pre- and post-intervention evaluations were performed in the morning after overnight PSG and in the last visit after the end of intervention, respectively. As for the primary outcome measure, the Pittsburgh Sleep Quality Index (PSQI) was adopted to assess subjective sleep quality [29]. Since there is no established definition for clinically significant treatment response in insomnia [30], we defined the positive treatment response as a decrease in PSQI score ≥3 points after intervention, based on several preceding insomnia treatment studies [30,31]. Different from these studies, however, we did not adopt the criteria based on sleep diary data due to the unavailability of data for some participants. The secondary outcome measures were the changes in the scores of Epworth Sleepiness Scale (ESS) [32], Beck Depression Inventory (BDI) [33], Beck Anxiety Inventory (BAI) [34], and Numerical Rating Scale for pain (NRS) [35].
Sleep diaries and actigraphy
Subjective and objective sleep parameters were assessed as secondary outcome measures. To obtain subjective sleep parameters, participants were asked to keep a sleep diary every day in the morning during the entire intervention period. The sleep diary contained items evaluating subjective sleep factors such as time in bed (TIB, the number of minutes spent in bed), sleep latency (SL, the number of minutes taken to fall asleep), wake after sleep onset (WASO, the number of minutes of wakefulness after sleep onset), total sleep time (TST, the number of minutes spent asleep) and sleep efficiency (SE, the ratio of TST to TIB). For examining the changes in subjective sleep quality before and after the treatment, we compared sleep parameters from sleep diaries during the pre- and post-intervention actigraphic recordings. We excluded the sleep diary data from participants whose answers were obviously unreliable, for example, “TST=0.” To confirm the adherence to the intervention protocols, the duration of daily device use was also recorded in daily sleep diaries.
For the assessment of objective sleep parameters, our study participants were requested to wear an accelerometer (wGT3XBT, ActiGraph, LLC, Pensacola, FL, USA) on their non-dominant wrist. Actigraphic monitoring was performed twice, before and after treatment. The pre- and post-intervention assessments were carried out for four days just before the start of intervention and for the last four days of the intervention period, respectively. The wrist actigraphy estimated sleep-wake status by capturing and recording physical activity and information regarding TIB was obtained from the daily sleep diary. ActiLife 6 software (ActiGraph, LLC, Pensacola, FL, USA) was used to analyze raw data and calculate the following four sleep variables: SL, WASO, TST, and SE. Data of participants who made proper use of actigraphy as instructed for at least 3 days for both times were included for the analysis.
Blood tests
The pre- and post-intervention blood tests were conducted as secondary outcome measures, concurrently with the questionnaire assessments. Blood samples were drawn from the antecubital vein in the morning (between 8 AM and 9 AM), in the fasting state more than 8 hours. They were properly processed and transported to the testing institute (Seoul Clinical Laboratories, Seoul, South Korea). We estimated the serum levels of interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-alpha), glucose, C-reactive protein (CRP), cortisol, and insulin since these blood biomarkers reflect inflammatory response and hypothalamic-pituitary-adrenal (HPA) axis activity [36,37].
Statistical analysis
The minimal required sample size was calculated as 128 subjects (64 for each group), using G*Power software at a confidence level of 95%, an effect size d of 0.5, and a statistical power of 0.8. Considering the drop-out rate of 20%, we decided to enroll more than 160 subjects (80 for each group). In this study, per-protocol (PP) analysis was performed, with exclusion of the data from those who had been dropped out and those with incomplete information regarding measurements of outcomes.
We compared demographic and clinical characteristics between the experimental and control groups, using the independent t-test for continuous variables and the chi-square test for categorical variables. Paired t-test or Wilcoxon signed rank test was adopted to examine the intra-group differences between pre-and post-intervention assessments including questionnaires, sleep diaries, wrist actigraphy, and blood tests. To evaluate the inter-group differences in the changes of parameters after treatment between the two study arms, we used analysis of covariance (ANCOVA) adjusted by age, sex, and baseline BMI. The exploratory sub-group analysis was performed after the patients were divided into two age groups according to several precedent studies on late-life insomnia: those aged >60 years and those aged ≤60 years [38-40]. Cohen’s d was calculated to estimate the effect size of the intervention in each group. All statistical analyses were carried out using SPSS version 25.0 for Windows (IBM Corp., Armonk, NY, USA) and a two-tailed pvalue of less than 0.05 was considered statistically significant.
RESULTS
Baseline demographic and clinical characteristics
A total of 196 patients suffering from insomnia disorder were enrolled in the study and 176 patients were randomized. Of those, 90 and 86 persons had been assigned to the experimental group and the control group, respectively. The dropout rate was 10.0% (9/90) in the experimental group and 8.1% (7/86) in the control group, with no significant difference between the two groups (p=0.795). Finally, 160 patients completed all the study protocols and were included for the analysis (Figure 1). When we compared the baseline demographic and clinical characteristics including objective sleep data from the PSG records, no statistically significant difference was found between the experimental and control group except for BMI and periodic limb movement index (PLMI). Compared to the control group, the experimental group showed significantly lower BMI and higher PLMI at the baseline assessment (Table 1). In addition, we found no significant difference in all baseline characteristics between those participants completed the study protocol and those who dropped out.
Safety reports
Tolerability data were available for 175 participants because one participant had dropped out before the first assessment for adverse events. Adverse reactions were reported by 35 patients (20%) at least once during the treatment period; headache (n=7), chest discomfort (n=2), myalgia (n=5), skin rash (n=3), urticaria (n=1), itching (n=9), tingling sensation (n=6), dizziness (n=3), epigastric pain (n=2), nausea (n=1), and fatigue (n=3). The symptoms obviously not related to the device use, such as common cold, gastrointestinal disturbances due to food intake, and pain induced by other underlying medical conditions, were not regarded as adverse reactions. Three patients had dropped out due to adverse events; two in the experimental group (tingling sensation and back pain) and one in the control group (shoulder pain). All the adverse reactions were tolerable and self-limiting within several days for remaining participants. Also, no significant difference was found in the occurrence of adverse events between completers and dropouts (19.4% [31/160] vs. 26.7% [4/15], p=0.500). Furthermore, among the completers, the incidence rate of adverse events did not differ significantly between the experimental and control group in the incidence rate of adverse events (17.3% [14/81] vs. 21.5% [17/79], p=0.498).
Effects of LF-TENS intervention
There was no substantial difference in the number of device use (24.05±3.19 days vs. 23.94±2.66 days, p=0.809) and the duration of daily use (53.74±6.12 minutes vs. 54.50±5.88 minutes, p=0.422) between the experimental group and the control group. The average current intensity of LF-TENS was 1.12± 0.13 mA at the neck and 1.13±0.13 mA at shoulder. Regarding rescue medication administration, those who used the active device showed slightly lower proportion of zolpidem use (34.6% [28/81] vs. 43% (34/79), p=0.272) and lower dosage of zolpidem (9.51±16.82 mg vs. 14.37±20.28 mg, p=0.100), although statistically not significant.
We tested the changes in the outcome measures before and after intervention period in the experimental and control groups among the whole study population. After four weeks of intervention, significant reductions of the PSQI, BDI, and BAI score were found in both study arms. Although the amplitude of these improvements was greater in the experimental group, it did not reach statistical significance (Table 2). The positive treatment response rate was 37.0% (30/81) in the experimental group and 30.4% (24/79) in the control group, yielding no substantial difference between the two groups (p=0.373) (Figure 2A). As for sleep parameters, both the experimental and control groups have reported an evident improvement in all the subjective sleep quality based on sleep diaries, without distinct betweengroup difference. In blood test results, meaningful differences before and after treatment were observed in serum cortisol levels both in the experimental and control groups, producing no significant difference in the changes of serum cortisol levels between the two groups. There was no profound change in actigraphy after study protocol in both study groups, without any noticeable between-group difference.

The comparison of outcome measures before and after 4 weeks of intervention among the whole participants
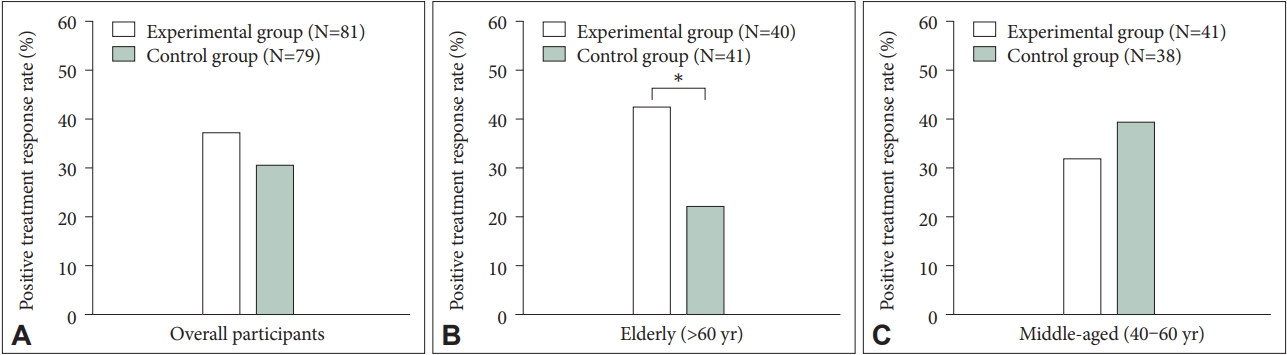
The positive treatment rate among study participants. A: Overall participants. B: Elderly participants (aged >60 years). C: Middle-aged participants (aged 40–60 years). *p<0.05.
Table 3 describes the effects of LF-TENS in the elderly patients over 60 years of age. We found a more prominent decrease of PSQI score in the experimental group (13.00±2.21 to 10.37±3.04) compared to the control group (12.15±2.59 to 10.95±2.70), after adjusting for possible confounders (p=0.039). Estimated effect size was much larger in the experimental group than in the control group (Cohen’s d=0.99 vs. 0.45). The rate of positive treatment response was also higher in the experimental group compared to those of the control group (42.5% [17/40] vs. 22.0% [9/41], p=0.048] (Figure 2B). Figure 3 represents the changes in PSQI score after intervention for each participant. Regarding blood test results, serum cortisol levels were significantly decreased in the experimental group (10.51± 2.90 μg/dL to 8.87±2.94 μg/dL, p=0.004) but not in the control group (10.58±2.66 μg/dL to 10.42±3.52 μg/dL, p=0.775), which also resulted in a substantial difference in the decrease of cortisol concentration between the two group (p=0.007). Although statistically insignificant, we found a trend-level decrease of serum TNF-alpha levels in the experimental group (0.92±0.32 pg/mL to 0.85±0.31 pg/mL, p=0.073), while an increase was observed in the control group (0.82±0.28 pg/mL to 0.88±0.39 pg/mL, p=0.131). Also, a significant inter-group difference was found between the two groups in the change of serum TNFalpha concentration (p=0.007). Compared to the control group, the experimental group showed larger estimated effect size in the serum cortisol (Cohen’s d=0.56 vs. 0.05) and TNF-alpha levels (Cohen’s d=0.22 vs. -0.18).

The comparison of outcome measures before and after 4 weeks of intervention among participants >60 years of age
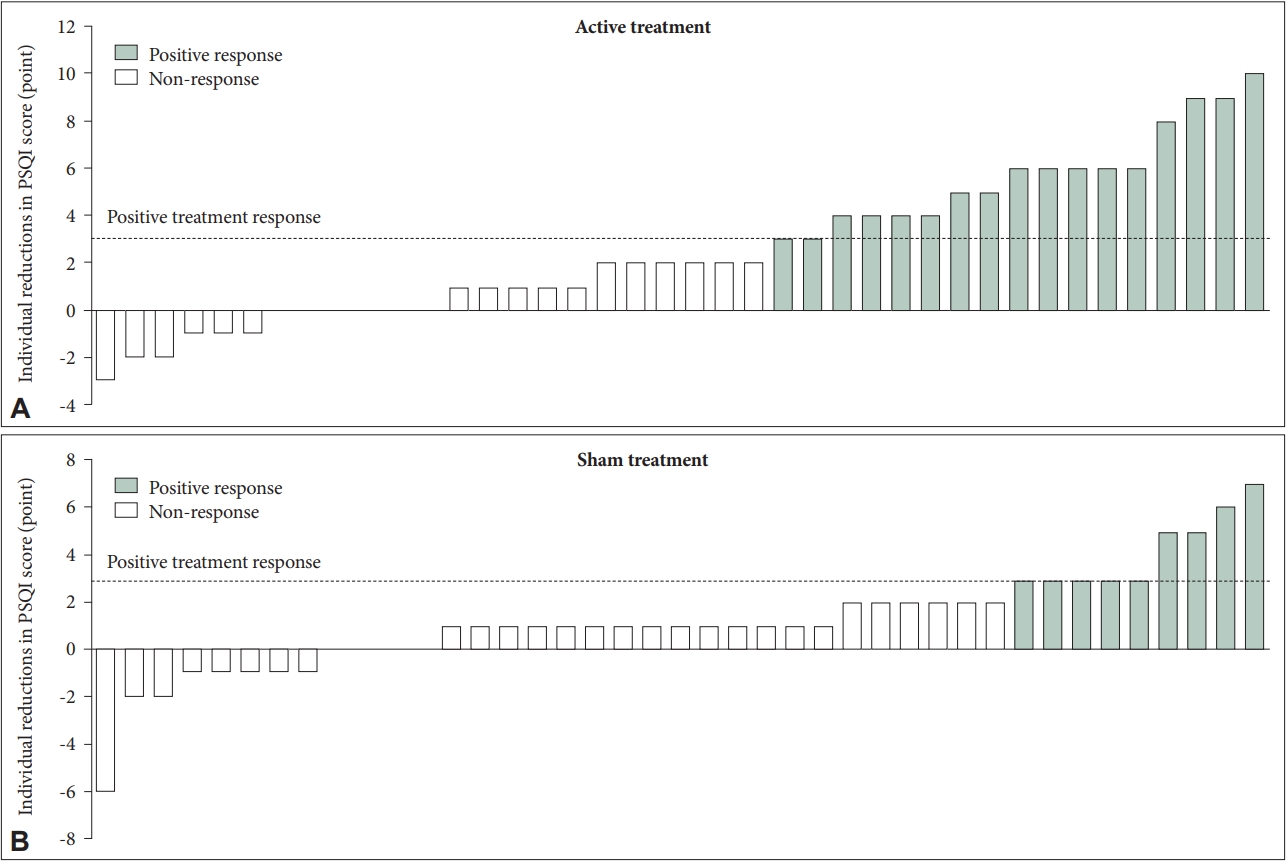
Individual distribution of the elderly patients aged >60 years with and without positive treatment response according to the reduction in Pittsburgh Sleep Quality Index (PSQI) score after intervention. A: The reduction in PSQI score after intervention in those with active treatment. B: The reduction in PSQI score after intervention in those with sham treatment.
Among the middle-aged patients aged 40 to 60 years, we could not find a statistically meaningful difference in the changes of outcome measures between the experimental and control groups (Table 4). In addition, no evident difference was observed both in the positive treatment response rate across the two groups (31.7% [13/41] vs. 39.5% [15/38], p=0.471) (Figure 2C).
DISCUSSION
In the current study, we found an improvement in subjective sleep quality and a higher treatment response rate among older insomnia patients over 60 years of age. Meanwhile LF-TENS was not effective for insomnia in the middle aged group (40 to 60 years). In the elderly patients, a significant decrease in serum cortisol level was observed in the experimental group exclusively. With regard to safety issues, no serious adverse reactions had occurred during the study period and the risk of adverse events of LF-TENS treatment did not differ from that of sham treatment.
In the present study, we selected bilateral upper trapezius muscles as application sites for several reasons. Since the trapezius muscles are innervated by spinal accessory nerve and cervical nerves C2 to C4 [41], the therapeutic effects of LF-TENS on trapezius muscles may affect CNS via this neuronal pathway. This muscle is also known to be related to sleep disturbances. A prospective study reported that an increased muscle response in the upper trapezius muscle could be a strong predictor of sleep complaints [42]. In addition, chronic trapezius myalgia might be associated with anxiety and depression [43], which may contribute to the aggravation of insomnia symptoms.
We observed the effects of LF-TENS on sleep quality among participants over 60 years of age while no significant treatment effect was found in the middle-aged group. This result might stem from the normal sleep physiology with aging and different manifestation of insomnia symptoms in older adults. A meta-analysis has revealed that total sleep time, sleep efficiency, and slow wave sleep decreased with age in the general healthy population [44]. The natural deterioration of sleep in the elderly can also generate anxiety about sleep and dysfunctional sleep habits, which may lead to poor subjective sleep quality and more severe sleep complaints. It is well-known that LF-TENS is helpful for muscular relaxation [45] and myorelaxation effects may play a role in the improvement of sleep quality since insomnia symptoms can be alleviated by the reduction of muscle tension and somatic arousal before bed [46]. Therefore, the effects of LF-TENS might be more prominent in the older insomnia patients due to their higher levels of sleep-related anxiety and stress response, compared to their younger counterparts.
The other potential physiological mechanism behind the effects of LF-TENS on insomnia among the elderly patients might be the modulation of HPA axis activity and inflammatory response. The existing literature has documented the bidirectional relationship between HPA axis hyperactivity and insomnia [47,48], and LF-TENS can down-regulate HPA axis activity since muscle relaxation that might be achieved with LFTENS, was reported to reduce cortisol secretion [49]. It is wellknown that cortisol acts as a marker of HPA axis function since it is released from adrenal gland in response to physical and psychological stress and its serum concentration is regulated by HPA activity [50]. On the basis of the aforementioned grounds, we hypothesized that serum cortisol levels would be decreased after LF-TENS treatment. In this study, we found a significant reduction in cortisol levels after intervention in the elderly patients, exclusively. This finding is in line with our results in the elderly patients, suggesting that the effect of LF-TENS on sleep quality in the elderly patients might be related to a decreased HPA axis activity. In addition, a decrease of TNF-alpha, a proinflammatory cytokine, might responsible for the improvement of insomnia since increase of daytime TNF secretion can cause fatigue and difficulty in falling asleep [51]. A study of Olaniyan and Ogunlade [52] also showed a significant decrease of TNFalpha after treatment of chronic insomnia, in consistent with our findings.
The strengths of this study include the large study population consisting of insomnia patients without medical and psychiatric comorbidities which may affect the sleep quality. Moreover, our study is the first randomized-controlled trial providing some evidence for the use of LF-TENS for the adjunctive treatment of insomnia among the elderly. Yet, the current study has several limitations to be considered. First, we conducted PP analysis, which may lead to an overestimation of treatment efficacy, compared to intention-to-treat analysis. However, our data reported relatively low drop-out rate (≤10%) in both study groups, which may reduce the possibility of selection bias. Second, the use of rescue medication may affect the results. In this study, zolpidem was allowed to prevent the serious deterioration of insomnia and there was no between-group difference in the use of rescue drug. Third, we cannot generalize our findings to young adults under 40 years of age or severe insomnia patients requiring ongoing treatment.
In summary, we found age-limited effects of LF-TENS for the improvement of subjective sleep quality in the elderly patients over 60 years of age. The modulation of HPA axis activity might be related to these effects of LF-TENS in the elderly insomnia patients. In addition, LF-TENS treatment was safe and tolerable without causing serious adverse events. Physicians might consider LF-TENS treatment as an adjunctive therapeutic option for insomnia of older adults.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
Seockhoon Chung, a contributing editor of the Psychiatry Investigation, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions
Conceptualization: Seockhoon Chung, In-Young Yoon. Data curation: Hyuk Joo Lee, Hayun Choi. Formal analysis: Hyuk Joo Lee. Funding acquisition: In-Young Yoon. Investigation: Hyuk Joo Lee, Jung Kyung Hong, Hayun Choi. Methodology: Hyuk Joo Lee, Hayun Choi, In-Young Yoon. Project administration: Hyuk Joo Lee. Resources: In-Young Yoon. Software: Hyuk Joo Lee. Supervision: Seockhoon Chung, In-Young Yoon. Validation: In-Young Yoon. Visualization: Hyuk Joo Lee. Writing—original draft: Hyuk Joo Lee, In-Young Yoon. Writing—review & editing: all authors.
Funding Statement
This work was supported by the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (grant number HI17C0203).



