Nature and Extent of Physical Comorbidities Among Korean Patients With Mental Illnesses: Pairwise and Network Analysis Based on Health Insurance Claims Data
Article information
Abstract
Objective
The nature of physical comorbidities in patients with mental illness may differ according to diagnosis and personal characteristics. We investigated this complexity by conventional logistic regression and network analysis.
Methods
A health insurance claims data in Korea was analyzed. For every combination of psychiatric and physical diagnoses, odds ratios were calculated adjusting age and sex. From the patient-diagnosis data, a network of diagnoses was constructed using Jaccard coefficient as the index of comorbidity.
Results
In 1,017,024 individuals, 77,447 (7.6%) were diagnosed with mental illnesses. The number of physical diagnoses among them was 11.2, which was 1.6 times higher than non-psychiatric groups. The most noticeable associations were 1) neurotic illnesses with gastrointestinal/pain disorders and 2) dementia with fracture, Parkinson’s disease, and cerebrovascular accidents. Unexpectedly, the diagnosis of metabolic syndrome was only scarcely found in patients with severe mental illnesses (SMIs). However, implicit associations between metabolic syndrome and SMIs were suggested in comorbidity networks.
Conclusion
Physical comorbidities in patients with mental illnesses were more extensive than those with other disease categories. However, the result raised questions as to whether the medical resources were being diverted to less serious conditions than more urgent conditions in patients with SMIs.
INTRODUCTION
Comorbidity relates to the simultaneous presence of two or more medical conditions at the same time [1]. In psychiatry, comorbidity is pervasive and of great importance in both clinical and theoretical aspects [2]. Clinicians are increasingly aware of the importance of physical comorbidities in the treatment of mental illnesses [1]. A study done in Korea reported that patients with any mental disorder have an increased risk of chronic physical conditions by an odds ratio of 1.5 to 2.8 [3]. A recent comprehensive study in Denmark estimated the hazard ratio for suffering physical illness after a diagnosis of mental illness to be 1.37 [4]. These ratios are expected to be even higher in patients with severe mental illnesses (SMIs) considering that they do not seek medical services on their own [5].
Physical comorbidity impedes recovery and restricts functional independence [6,7]. Patients with SMIs even have a reduced lifespan [8], which is partly attributed to comorbid medical conditions [9]. Therefore, it is clinicians’ responsibility to detect comorbid conditions and effectively manage them [10].
However, the fact that someone has been given multiple diagnostic labels does not entail that he/she is actually suffering from all those illnesses. An understanding of the phenomenon of comorbidity cannot be completed without considering individual health-seeking behavior and regional healthcare systems. Patients with anxiety or depression often seek consultation from medical specialists before receiving proper psychiatric diagnosis. This is one of the main reasons behind treatment delays and inefficient usage of healthcare resources [11]. Patients’ own denial, limited medical information, and the clinicians’ narrow perspective limited to their own specialties could extend the list of unnecessary diagnostic labels.
So far, studies on physical comorbidity have mainly focused on depression and SMIs [12]. They have emphasized the elevated risk of so-called lifestyle diseases, such as metabolic syndrome, cardiovascular diseases, and cerebrovascular accidents. The common data sources for existing studies were cohort studies, epidemiological catchment area survey or review of admission records. These studies were able to calculate relatively accurate comorbidity rates for preselected pairs of diagnoses because they employed well-designed survey tools or laboratory tests [13-15]. Despite this, they failed to address all the combinatorial pairs of diagnoses that were not included in the study design.
Second, comorbidity in a strict biological sense is not the same as comorbidity in the sociomedical dimension. The latter deals with what problems patients with a certain disease mainly complain about, how they interpret them, which medical specialists they consult and what diagnoses they receive in this process [16-18]. Finally, the results obtained from traditional studies only describe a dyadic relationship in which one disease increases the risk of another. However, according to the concept of triadic closure from graph theory, if the connections A-B and B-C exist, there is a tendency for the new connection A-C to be formed. Therefore, to understand the triadic or multi-adic relationship among the assorted set of diagnoses, it is advantageous to borrow the technique of network analysis [19].
To facilitate such research, large-scale epidemiological data and detailed quantitative analysis is mandatory. Health insurance claim database is a useful data source with a long history and a solid theoretical foundation [20,21]. The number of studies utilizing such big data is steadily increasing. Comorbidity rates are being aggregated for millions or tens of millions of individuals with the aid of national registry or claims data. However, since such datasets are not readily accessible in most countries, the published results are confined to some European nations [2,4,22]. Large-scale studies investigating comorbidity patterns are still scarce in Asia [12].
In this study, we sought clues to the following clinically relevant questions by analyzing the health insurance claims dataset in Korea: Is the physical comorbidity in psychiatric patients more extensive than in other disease categories? Which psychiatric diagnoses are associated most closely (or least closely) to physical conditions? What are the physical conditions they have? Are there specific physical conditions associated with each psychiatric diagnosis? Does the extensive physical comorbidity reflect the health-seeking behavior of neurotic patients or the actual health risk of patients with SMIs?
The obtained result will provide supporting data to help understand the complex nature and extent of physical comorbidity in psychiatric patients. In addition, by revealing the personal traits of Korean patients, cultural influence they receive and the limitations of the current healthcare delivery system, it will pose challenges for Korean psychiatrists to design better ways to help patients with mental illnesses.
METHODS
Data source
In Korea, the National Health Insurance System (NHIS) is a mandatory social insurance service. The health insurance claim data were collected and archived by the Health Insurance Review and Assessment Service (HIRA). A part of this huge dataset is annually published to facilitate the openness of governmental data. This dataset is freely accessible without restriction at a government operated data portal (https://data.go.kr/data/15007115/fileData.do).
This annual data consisted of crude demographic information and the medical claims records for randomly sampled 1 million subscribers. A sufficiently large sample size and the random sampling procedure assure the representativeness of the dataset. It was carefully anonymized, and personal identifiable information had been erased. It contains diagnoses, the start and end date of medical consultation and the number of prescription days. The diagnoses were coded in the Korean Standard Classification of Disease, version 7 (KCD-7), which is an adapted version of the International Classification of Diseases, version 10 (ICD-10). Due to privacy concerns, the detailed code of the psychiatric diagnosis is no longer provided since 2016. Therefore, this study was conducted based on data from 2015.
The study design and the data analysis protocol were reviewed and approved by Institutional Review Board of Eulji University Hospital (EMC 2021-04-025).
Data processing
The raw data was imported into a database management system called Neo4J. Neo4j is a database specifically designed to store and query graph type datasets. Recently, with the emphasis on the graph properties of biological data, Neo4J is being widely used in biomedical research [23-25].
Neo4J provides a structured query language-inspired query language called Cypher to navigate the stored data. Several in-house Cypher queries were written by authors to address the research questions. In the raw data, each record indicated the patient’s single claim for the medical consultation. If the patient had sought treatment for different problems, another record for a separate claim had been added. Each record contained a couple of diagnoses made by the treating physician. Thus, each patient has multiple records of medical claims and each record has multiple diagnoses. These patient-diagnosis links were stored as a bipartite graph with two types of nodes (patient and diagnosis). A comorbidity network was built by projecting the bipartite graph into a unipartite graph with Jaccard similarity coefficients as their link weights.
Calculation of the degree of comorbidity
The degree of comorbidity at the individual level was represented by the number of distinct diagnoses each patient had during the study period. Since this study mainly focused on the physical comorbidity in psychiatric patients, additional psychiatric diagnoses were excluded when counting the comorbid conditions.
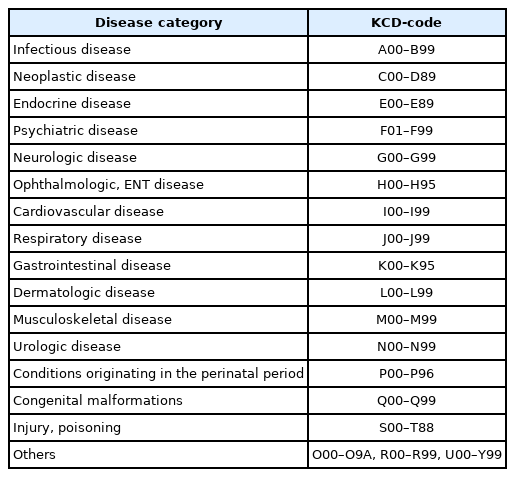
All patients were classified into separate disease categories. In Table 1, the KCD-codes were mapped to conveniently chosen disease categories used in the study. As subjects had been diagnosed with one or more conditions, he or she could belong to more than one category. The individual degree of comorbidity was averaged to obtain the degree of comorbidity at the disease category level. After that, the disease categories were ranked according to their degree of comorbidity. Similarly, F-code diagnoses (psychiatric diagnoses) were ranked according to the diagnosis level degree of comorbidity.
Evaluation of disease-disease association
The likelihood of two diagnoses being associated can be affected by many confounding factors including their overall prevalence. Therefore, simply counting the number of comorbid conditions for each psychiatric diagnosis could not provide an unbiased estimate of comorbidity. As the data contained only sex and age as the available demographic variables, pairwise logistic regression was conducted for all possible diagnostic pairs (between psychiatric and physical diagnoses) with sex and age as confounding variables.
The magnitude of the adjusted odds ratios (AORs) and associated p-values were used to select significant associations. Since the number of required pairwise logistic regression was over one million, the level of significance was adjusted by Bonferroni correction (p<10-7 since the usual p-value 0.05/106= 5×10-7) to ensure that spurious associations were not considered significant solely due to the huge sample size [26].
We paid attention not only to the positive comorbidity (AOR>1), but also to the negative comorbidity (AOR<1) [27]. It is unconvincing to argue that the negative comorbidity implies that a certain disease affords protection to another set of diseases. However, the negative comorbidity obtained in health claims data may be indicative of systemic under-recognition or ignorance of a certain set of physical conditions.
Construction of a comorbidity network
A comorbidity network was built by projecting a bipartite network (patient-disease association) into an unipartite network (disease-disease association) [28]. Jaccard coefficient was used for the edge weight which represented the comorbidity (=similarity) of the two diseases. Existing literature disagrees on what indicators are the most appropriate for representing the similarity of a pair of nodes. Recommended indicators include 2×2 contingency table-based measures such as odds ratio, relative risk, or observed-to-expected ratio (O/E ratio) [26,29,30]. However, these indicators have some disadvantages when trying to construct and visualize a network. First of all, they are not bounded and can extend to positive infinity. Although logarithmic transformation could in part remedy this problem, it still is unbounded and permits few large values [31]. In networks, few larger values of similarity may dominate the layout and prevent the global structure from emerging.
Another issue is the lack of information in the data source. In a pre-planned study, rating scales, structured interviews, or laboratory tests would be used to unequivocally determine the presence or absence of a target disease. From these, all four cells of the contingency table and thus the marginal distribution could be known without any uncertainty. On the other hand, claim based data like ours do not provide any information on individuals who did not seek medical consultation. Since not receiving medical treatment and not having a disease are two separate issues, the marginal distribution cannot be known exactly. Therefore, any contingency table-based measures had to suffer from this lack of information. By contrast, overlap-based measures, such as Jaccard coefficient, do not require the marginal distribution, which is why they are commonly used in gene-sharing networks. Such networks have been traditionally called Comorbidity Network, Phenotype Disease Network, Human Disease Network, or Disease Similarity Network [32-36].
The distribution of the obtained Jaccard coefficients was highly right-skewed, such that over 90% of them were almost zero. In addition, the number of edges in the whole network was too large (=381,714), so neither the backbone structure extraction nor the visualization were possible. To make it work, it was arbitrarily decided that the size of the network had to be reduced to less than 1%. So, the weak links with the coefficient less than 1 percentile of the whole distribution (Jaccard coefficient= 0.025) were discarded. The reduced network was visualized by the quadrilateral backbone layout algorithm [37]. It tries to reflect the relative edge strength as much as possible and uncover the potential community structures. Undoubtedly, it is not recommended to regard the 2D layout distance as the literal measure of connectedness, but it is still possible to interpret that closely positioned nodes are more similar and belong to the same community. In this way, the global structure of the network can emerge and provide a birds-eye view of disease-clustering patterns. Diseases within close proximity can potentially share important characteristics such as risk factors, pathophysiology, or treatment strategy [34].
All the statistical analyses were conducted by the open source statistical package R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria). Network analysis and visualization were specifically aided by the R packages “tidygraph” and “ggraph.”
RESULTS
Characteristic of the dataset
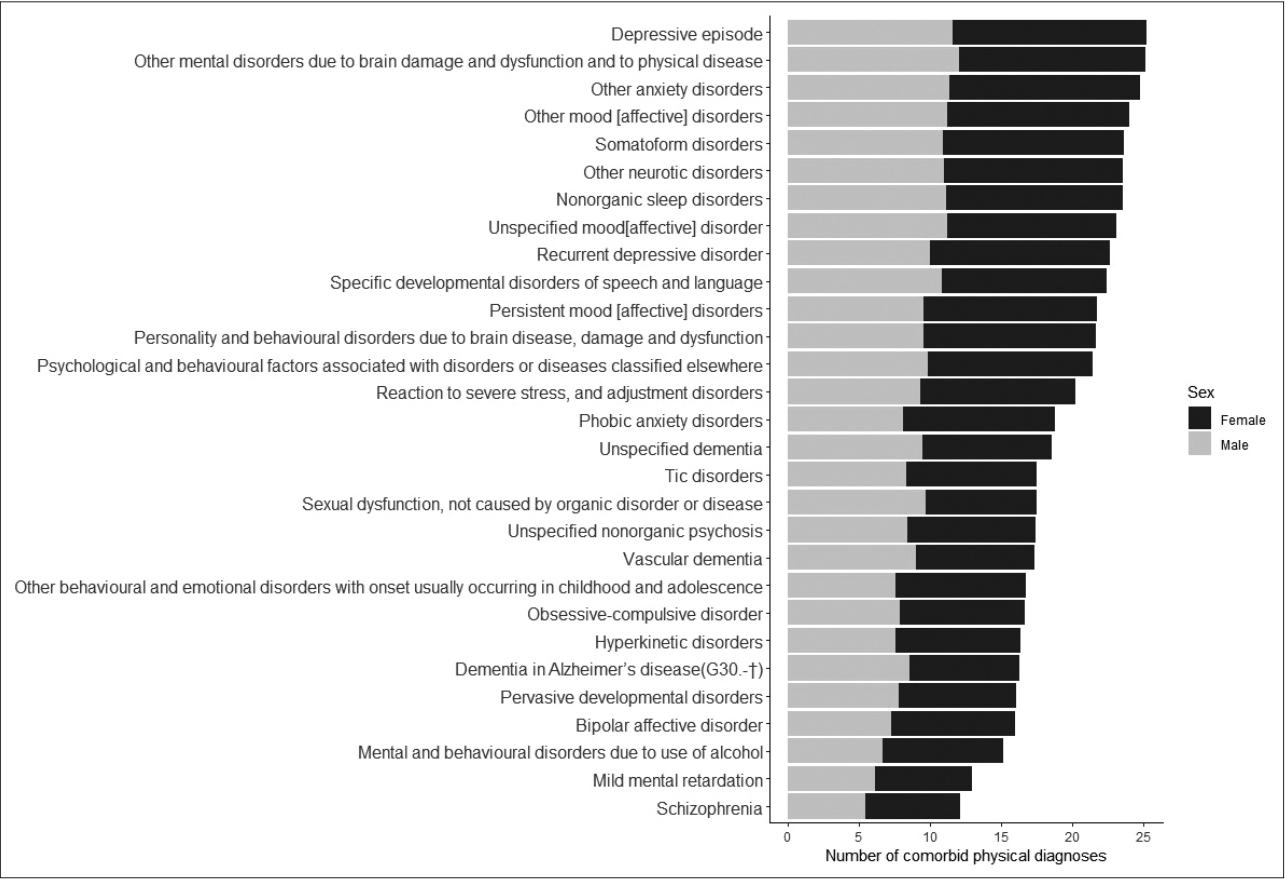
The dataset contained the health claim data for 1,017,024 individuals (486,725 males and 530,299 females). The total number of medical claims was 11,231,930, such that each individual received, on average, 11 outpatient or inpatient treatment. The sex and age distribution of the subjects were shown in Table 2. The percentage of subjects with one or more psychiatric diagnoses (F00–F99) was 7.6% (77,447). Their age and the proportion of female subjects were greater than the rest. A total of 77 distinct psychiatric diagnoses had been made to these patients. The most prevalent psychiatric diagnoses were depressive episodes (26.4%), other anxiety disorders (25.9%) and sleep disorders (9.2%).
The degree of comorbidity in various disease categories
The included subjects had an average of 7.5 distinct diagnoses (8.2 for females and 6.7 for males) regardless of disease category. For psychiatric patients, the mean number of distinct diagnoses was 11.7 (12.4 for females and 10.6 for males). Apart from the index diagnosis (an arbitrarily chosen single psychiatric diagnosis), additional diagnoses were separated into 1) other psychiatric diagnosis (F-code) and 2) diagnoses related to physical conditions (non–F-code). While the average number of additional F-code diagnosis was only 0.5, that of comorbid physical diagnosis was 11.2 (11.8 for females and 10.1 for males).
In order to determine whether psychiatric patients had a higher number of comorbid diagnoses than the rest, the mean numbers of comorbid diagnoses (including index diagnosis) across different disease categories were compared and displayed in Figure 1.
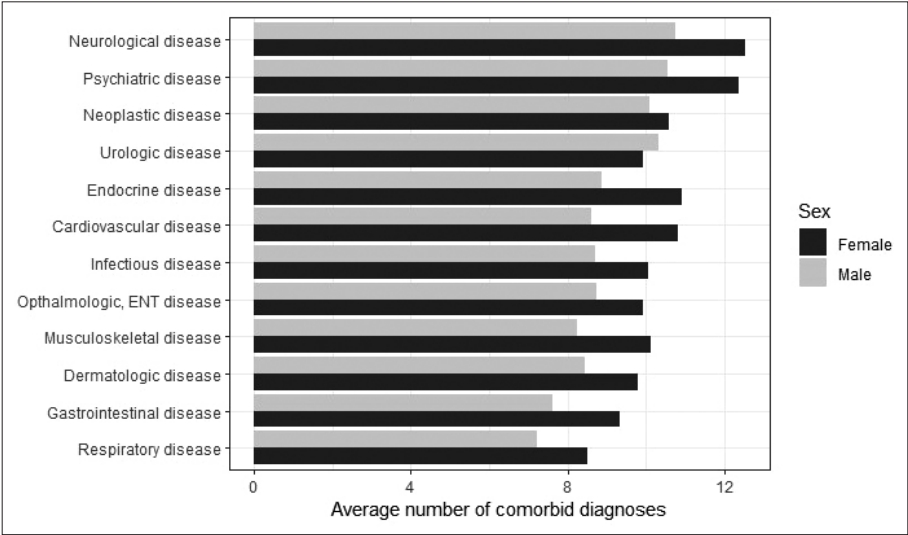
The average number of comorbid diagnoses in subjects belonging to different disease categories. The largest number of comorbid diagnoses was associated with neurological diseases. Common to all categories (except urologic disease), female subjects had a lot more comorbid diagnosis. ENT, ear, nose and throat.
The category with the largest number of comorbid diagnoses was neurological disease, which was closely followed by psychiatric disease. Compared to them, other disease categories had a relatively fewer number of comorbid diagnoses. To confirm this difference, Poisson regression was performed. The presence of psychiatric diagnosis significantly elevated the number of comorbid diagnoses (b=0.47; t(1,017,019)=229.85; p<10-7; 95% confidence interval, 1.60 to 1.61) adjusted for sex and age. According to this model, having psychiatric diagnoses increases the number of comorbid diagnoses by 1.6 times.
The number of comorbid diagnoses in each psychiatric diagnoses
The average number of comorbid physical diagnoses for each psychiatric diagnosis was displayed in Figure 2. Other mood disorders (F38), other mental disorders due to brain damage (F06), other neurotic disorders (F48), somatoform disorders (F45), unspecified mood disorder (F39), and other anxiety disorders (F41) were the diagnoses with the highest degree of physical comorbidity. In contrast, mental retardation (F70, F71), schizophrenia (F20), habit and impulse disorders (F63), schizoaffective disorders (F25) were the diagnoses with the lowest degree of comorbidity.
Physical diagnoses of which prevalence is increased or decreased by comorbid psychiatric diagnosis
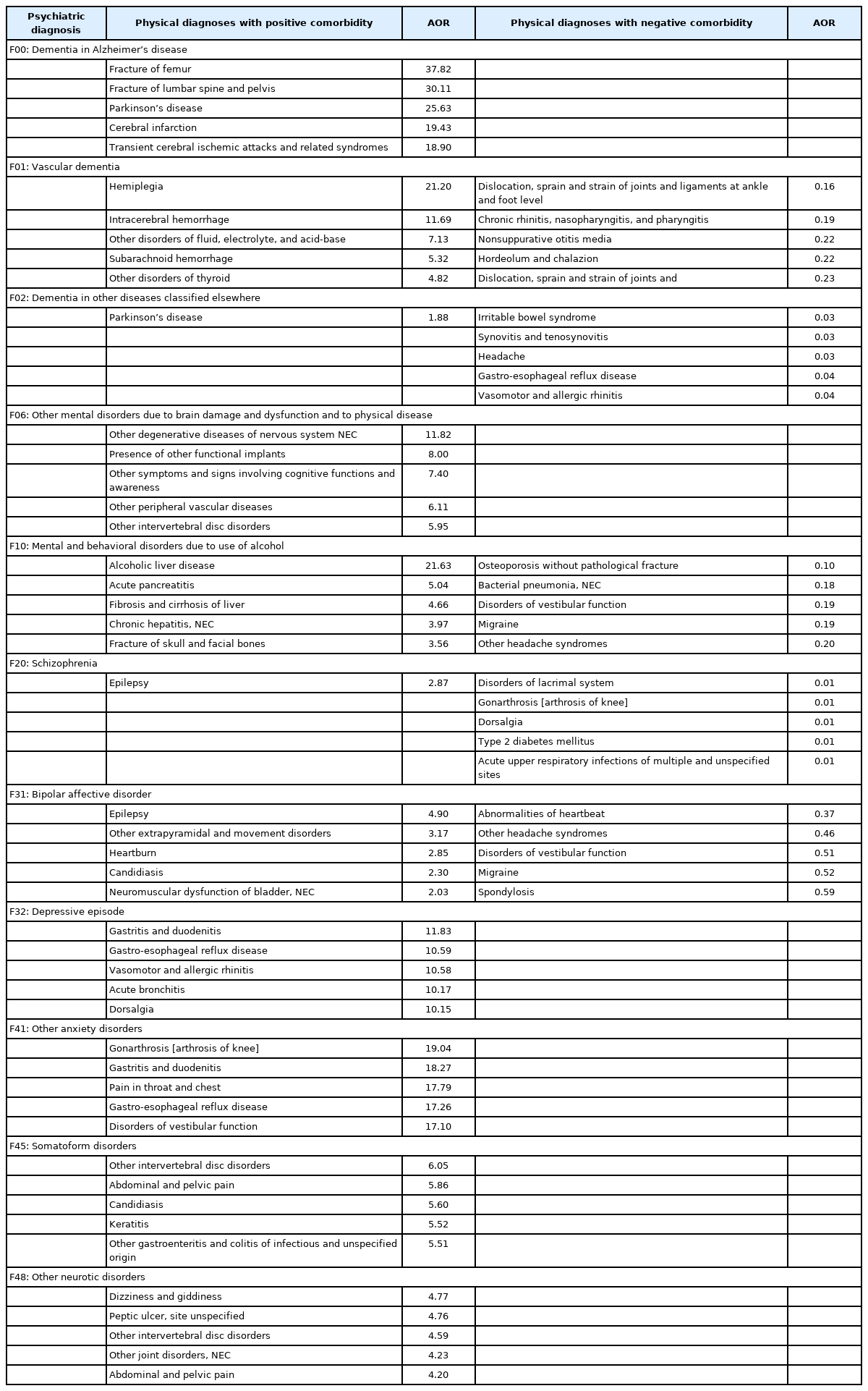
The number of physical conditions which had at least once been co-diagnosed with any psychiatric illness was 820. As there were 77 F-code diagnoses, the total number of unique combinations to be examined was 63,140 (=77×820). For each combination, a logistic regression was performed controlling for the effect of age and sex to obtain AORs. Some of the main findings of the result were listed in Table 3. The leftmost column listed representative psychiatric diagnoses. The middle column listed the physical diagnoses that had a positive comorbidity relationship with the former. Up to 5 diagnoses with the highest odds ratios were selected among the comorbid combinations that had passed statistical significance (p<10-7). The fourth column presented the physical disorders that were less likely to be found with each psychiatric diagnosis (negative comorbidity). As before, up to 5 diagnoses with the smallest odds ratio were selected among the statistically significant connections. Some of the cells contained less than 5 diagnoses or nothing because the majority of the comorbid combinations for that particular diagnosis didn’t pass the statistical tests.
Comorbidity network
The comorbidity network is visualized in Figure 3. According to a visual inspection, two groups of psychiatric diagnoses could be discerned: 1) On the left side of the figure, a group of child/adolescent psychiatric diagnoses was positioned near the perinatal and congenital conditions. In the central area, another group of psychiatric diagnoses was located intermingled with other physical conditions. Closer inspection suggested that they could be divided into two sub-groups. The first one (numbered 2-1) included both SMIs and various types of dementia. These diagnoses displayed close connections with cardiovascular and neurological conditions. The second (numbered 2-2) was mainly composed of depression (F32, F33), anxiety (F41) and organic mental disorder (F06). They were closely linked with musculoskeletal, gastrointestinal (GI) and injury related conditions. Those injuries included minor ones like sprain, falldown and fracture and were not related to suicidal attempts.

The network structure of comorbidity relationships among the diagnoses belonging to different disease categories. Psychiatric diagnoses were marked with larger violet circles and labeled with corresponding KCD-7 codes. They were also divided into three distinct groups (1, 2-1 and 2-2) according to their comorbid relationship with other diagnoses: 1) Group 1 consisted mainly of child-adolescent diseases, 2) group 2-1 of severe mental illnesses and dementia, and 3) group 2-2 of neurotic illnesses. The insert is a magnified view of the region containing severe mental illnesses (schizophrenia, schizoaffective disorder, and bipolar disorder etc.). In this magnified view, several endocrine diseases can be discerned (E10: type I diabetes mellitus, E14: unspecified diabetes mellitus).
DISCUSSION
In the present study, the comorbidity relations between psychiatric illnesses and physical conditions were explored using large-scale health claims data. Patients with psychiatric diagnoses had the second highest number of comorbid physical conditions, surpassed by neurological diseases. The high comorbidity was mainly driven by mood/anxiety disorders, somatoform disorders, and organic mental disorders. The degree of comorbidity was deeply influenced by age and sex. After adjusting the effect of age and sex, lists of physical conditions with significantly elevated or lowered odds ratio for each psychiatric diagnosis were obtained. Several noticeable associations were 1) neurotic illnesses with GI and pain disorders, 2) dementia with fracture, Parkinson’s disease and cerebrovascular accidents, 3) schizophrenia and bipolar disorder with epilepsy, and 4) alcohol use disorder with liver and pancreatic diseases. As to the negative comorbidity, schizophrenia was associated with a much lower prevalence of type 2 diabetes mellitus, quite contrary to prior expectation.
The phenomenon of comorbidity is often interpreted as comorbid diseases sharing a common pathophysiology or overlapping risk factors [38]. Chronic stress induces proinflammatory state, overactivation of the hypothalamic-pituitary-adrenal axis, or elevation of sympathetic tone, thus causing depression as well as various lifestyle diseases [39-41]. It helps to explain why the most frequent physical diagnosis with depression and neurotic illnesses were common GI troubles and musculoskeletal pain disorders. The connection between neurotic illnesses and GI trouble may be explained by the tight connection between emotion and GI. According to a Swedish study exploring the predictors of GI symptoms in a large sample of young psychiatric patients, the severity of depressive symptoms, trait anxiety and stress susceptibility were the independent predictors of the Gastrointestinal Symptom Rating Scale for Irritable Bowel Symptom [42]. The author attributed this finding to the bidirectional connection between the gut and brain via vagus nerve, enteric nervous system and alteration of gut flora, commonly referred to as gut-brain axis.
Neurotic illnesses can also lower the threshold of pain perception and promote hypochondriacal worries. In a long-term follow-up study of a community sample, depression was found to be the precursor to future back pain diagnoses [43]. Patients with somatoform disorder often exhibit a heightened focus on their own bodies, and catastrophically interpret their bodily sensation as a separate illness requiring urgent intervention [44]. Some neuroimaging studies even provided a neurobiological basis for this somatosensory amplification phenomenon [45,46].
Apart from these biological explanations, it is necessary to consider psychological, social, and cultural factors, especially when interpreting comorbidity in claims data [26]. The claims data kept the record only if the patient was aware of the problem and sought medical consultation. Therefore, it may reflect how the patient perceived and interpreted the problem, and which specialists he/she wanted to consult. It is not uncommon for general practitioners and medical specialists to overlook the possibility of mental illness and diagnose somatic accompaniments of a mental illness as physical diseases [47,48]. It may lead to an inflated rate of comorbidity [49]. The observation that patients with neurotic illnesses had an unusually large number of comorbid diagnoses indicate that they might have erroneously recognized their somatic symptoms as physical problems [50-52]. Some of the comorbid diagnoses might actually be misdiagnosed and contribute to treatment delays or inefficient use of healthcare resources. Underrepresentation of serious conditions like hypertension, metabolic and neoplastic diseases gave weight to this interpretation. Regardless of the specific psychiatric diagnosis, nonspecific GI, respiratory, musculoskeletal and sleep-related symptoms are often the primary cause of medical consultations and also the predominant reason why these patients visit primary care physicians. A lot of these symptoms are medically unexplained and an assortment of diagnostic labels are attached reflecting this inexplicability.
This distorted situation was also evidenced by the finding that the odds ratios of metabolic and cardiovascular diseases were not elevated but even lower than expected (negative comorbidity) in patients with SMIs. Crump et al. [53] reported that patients with schizophrenia were three times more likely to die of ischemic heart disease or cancer, but the diagnosis rate of these conditions was not elevated in nonfatal cases. Even in fatal cases, these patients were less likely to have been previously diagnosed with these conditions. This issue of under-recognition and under-treatment had also been discussed in a study with the Scottish population [5]. Although patients with schizophrenia had a higher number of physical morbidities, the recorded rates of hypertension and cardiovascular diseases in them were lower than the control group. People with mood and anxiety disorders reported significantly more contact with medical specialists for somatic diseases, but patients with schizophrenia did not receive enough care [54]. Notably, it depends much on the culture and healthcare delivery system of each region. Our results indicated that the current status of the medical system in Korea is not ideal for serious conditions that can compromise physical health and life expectancy of patients with SMIs.
Korea provides better access to health care than other countries, because the government-sponsored health insurance system (NHIS) covers most expenses. As the referral process from a primary physician to a large university hospital is neither complicated nor regulated, patients can receive whatever service they desire from any specialist. While such a system may seem ideal, the higher affordability and accessibility can result in overdiagnosis and overtreatment, exemplified by the recent epidemic of thyroid cancer in Korea [55]. Another issue is that in order to be reimbursed by the insurance system, a plethora of ad-hoc diagnoses must be made. Therefore, even if the primary physician notices that it is an incidental symptom accompanying depression, he/she has to make a separate physical diagnosis.
In contrast, Korean patients with SMIs are less likely to use mental health services due to social prejudice, stigma, as well as lack of insight. Korea’s mental health system does not yet offer mature community services, and inpatient treatment is overused, leaving patients with delayed diagnoses hospitalized for an extended period of time and disconnected from society as a result [56]. Even after discharge, they are not seamlessly integrated into the local community, and unless managed by case managers, they are less likely to receive multifaceted treatment other than just antipsychotic treatment. A possible explanation for the unexpectedly low rate of seeking treatment for lifestyle diseases in SMI patients may be related to their being isolated from society and sequestered in a blind spot of the healthcare system.
Mental health professionals working in the community also need to be alert. In particular, middle-aged and older patients with chronic mental illness are at risk of losing the attention of clinicians because their psychiatric symptoms are not prominent. Caretakers are also getting older and cannot afford to provide proper care for them. For example, it was reported that the rate of medical check-ups of patients with SMIs was much lower than that of the healthy control [57]. It may have been due to their low level of health literacy, but not enough recommendations or encouragement from mental health professionals may have also played a part.
We included a comorbidity network for a more advanced analysis of comorbid relations. It showed that there was differential connectedness between diagnoses, resulting in a complex grouping structure. Diagnoses belonging to the same disease category tended to cluster together. However, this was not always the case, and there were many regions where diagnoses belonging to different categories mingled together to form a group of heterogeneous components. The two prominent findings were 1) SMIs and dementia are closely located with cardiovascular and neurological conditions and 2) neurotic conditions such as depression, anxiety, and somatoform disorder were tightly linked with musculoskeletal, GI, and injury related conditions. Also of interest was the finding that these latter diagnoses (musculoskeletal, GI, and injuries) were inseparably linked.
Findings from the network analysis generally corresponded with the results obtained from pairwise comorbidity analysis. Even so, the indirect links between psychiatric and metabolic disorders could be discerned only in network analysis. The sub-group 2-1) had three diabetes diagnoses (E10: type 1 diabetes mellitus, E13: other diabetes mellitus, E14: unspecified diabetes mellitus; Figure 3), while the sub-group 2-2) had two adult-onset metabolic diseases (E11: type 2 diabetes mellitus, E78: dyslipidemia). By any measure, such links with endocrinological diagnoses could not be found in pairwise analysis. Patients’ unwillingness, especially patients with SMIs, to seek treatment on their own may have obscured latent comorbidities in the pairwise analysis.
Examining comorbidity from a network perspective may present a different picture than from a pairwise analysis. One such difference is that the latter cannot not address the indirect comorbidity mediated by intervening associations with other diseases [58]. The comorbidity network can deepen the understanding of multi-dimensional aspects of disease-disease correlation, such as exposure to risk factors, diagnosis and treatment, health psychology, and determinants of better or worse prognosis [34].
The limitation of this study came from the nature of available data. The claim data only recorded the patients’ seeking treatment voluntarily or inevitably. Therefore, omission in the data did not necessarily mean the absence of disease. Although the dataset provided insight into patients’ health-seeking behavior and physicians’ diagnosis practice, it was not suitable for the precise calculation of comorbidity. In addition, the diagnostic label used in this study may not reflect the actual patients’ condition. In Korean medical systems, diagnostic labels are assigned for various purposes. For example, KCD codes for epileptic syndromes may have been given to avoid reduction in insurance reimbursement for mood stabilizers and, likewise, codes for depressive disorders may have been used to justify antidepressant prescriptions.
Another shortcoming was that the data were only a snapshot of a fixed period. Cross-sectionally measured comorbidities may not have much meaning in itself. It cannot answer the questions as to which set of diagnoses in the present lead to which set of diagnoses in the future. Analyzing the temporal order of the diagnoses with longitudinal data may provide evidence for the cause-effect relationship. Several studies addressed this issue and tried to delineate the pattern of disease progression [29,30,59,60]. They are challenging tasks since access to sensitive datasets is restricted and the methodology to analyze dynamic networks is refined. Besides, temporal order does not necessarily guarantee the causal relationship. The authors of a nation-wide longitudinal study, which examined the diagnostic history of patients over many years, could not determine whether mental illness had been the cause of the following physical illnesses [4]. With this indeterminacy of the causal or temporal direction, it was also hard to decide whether the physical conditions accompanied the psychiatric disorders or vice versa. The result in this study could also be interpreted as psychiatric comorbidities in medical patients. Analysis from this alternate perspective would need a separate study.
In this study, we quantitatively analyzed the degree and nature of physical comorbidity in psychiatric patients. With the help of large-scale health claims data and network-based analysis methods, we could derive several insights from the analysis results. The results obtained are expected to remind psychiatrists that patients are receiving treatment for somatic symptoms from various medical specialists with diverse interpretations. In addition, they would help policy makers to formulate the efficient use of medical resources and medical delivery systems.
Notes
Availability of Data and Material
The datasets analyzed during the current study are publicly available at a Korean government operated public data portal (https://data.go.kr/data/15007115/fileData.do).
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Seong Hoon Jeong. Data curation: Seong Hoon Jeong. Formal analysis: Seong Hoon Jeong. Investigation: Seong Hoon Jeong. Methodology: Seong Hoon Jeong. Project administration: Ho Joon Kim. Software: Seong Hoon Jeong. Supervision: Seong Hoon Jeong. Validation: Sam Yi Shin. Visualization: Seong Hoon Jeong. Writing—original draft: Ho Joon Kim. Writing—review & editing: Seong Hoon Jeong, Sam Yi Shin.
Funding Statement
None