Bupropion for Interferon-Alpha-Induced Depression in Patients with Hepatitis C Viral Infection: An Open-Label Study
Article information
Abstract
Interferon (IFN)-α therapy for chronic hepatitis C virus (HCV) infection is frequently associated with major depressive episodes. Bupropion, a commonly used antidepressant agent, has recently found to have strong anti-inflammatory effects in animal models. Despite of the theoretical relevancy, the antidepressant effect of bupropion in IFN-alpha-induced depression has never been studied. Ten HCV patients with IFN-α-induced depression were recruited to receive 8-week bupropion treatment and were assessed every 2 weeks for depressive symptoms by the Hamilton rating scale for depression (HAMD) and somatic symptoms by the Neurotoxicity Rating Scale (NRS). Four of the 10 patients met the criteria for remission (total HAMD scores≤7), and 5 patients met the criteria for response (at least 50% reduction in total HAMD scores). In addition, 5 patients had 50% decreases in NRS for neuropsychiatric symptoms. This preliminary open-label study suggests that bupropion is effective in treating IFN-alpha-induced depressive and somatic symptoms.
INTRODUCTION
The current standard treatment for patients with chronic hepatitis C viral (HCV) infection is the cytokine therapy with interferon-alpha (IFN-alpha). However, IFN-alpha based therapy is associated with severe neuropsychiatric symptoms, including the development of major depressive episode (MDE), and other symptoms that resemble cytokine-induced "sickness behaviour."1,2 Selective serotonin reuptake inhibitors (SSRIs) are effective in the management of patients with INF-alpha-induced depression,3,4 especially for mood and cognitive symptoms. However, the somatic symptoms such as fatigue and anorexia are less responsive to SSRIs treatment.3 In addition, SSRIs also have been reported to develop manic switching.5 In fact, the anti-inflammatory agents, including non-steroidal anti-inflammatory drugs and cyclo-oxygenase-2 inhibitors, have been commonly used to treat neurovegetative somatic symptoms during IFN-alpha therapy in clinical settings.6
Bupropion is a norepinephrine (NE) and dopamine (DA) reuptake inhibitor antidepressant agent and has been reported to be effective in the treating major depressive disorder.7,8,9 Interestingly, bupropion exerted strong anti-inflammatory effects of down-regulating tumor necrosis factor-alpha, interleukin-1beta, and IFN-gamma.10 Despite of the theoretical relevancy, the effect of bupropion on depressive and somatic symptoms in IFN-alpha-induced depression has never been studied.
METHODS
We conducted this open-label study to evaluate the effect of bupropion on IFN-alpha-induced depressive and somatic symptoms in patients with HCV infection. Since year 2005, a psychiatric team has been working together with the hepatologists to provide a combined care for HCV patients referred to IFN-α therapy at the Liver Centre of China Medical University Hospital, Taichung, Taiwan. The institutional ethics review board approved this research before it underwent between July 2006 and June 2009. Patients with chronic HCV infection were assessed by the hepatologists to receive peg-IFN-alpha-2β (1.5 µg per kilogram of body weight, subcutaneously, once weekly) and daily ribavirin (800-1200 mg). Eligible subjects were those who developed the Diagnostic and Statistical Manual of Mental Disorders-fourth edition (DSM-IV) criteria of MDE during IFN therapy. After fully explained about all the possible treatment alternatives, the patients who signed informed consent were enrolled to start with bupropion treatment in 150 mg/day. The dosage could be increased according to response or tolerability based on the clinicians' clinical judgment. Participants were assessed every 2 weeks by independent raters with the 21-item Hamilton Rating Scale for Depression (HAM-D) for depressive symptoms, the Neurotoxicity Rating Scale (NRS) for somatic symptoms, and the Chalder Fatigue Questionnaire (CFQ) for fatigue symptoms. The laboratory examinations, including hepatic enzymes (AST/ALT) and viral mRNA titers, and the adverse effects associated with IFN and bupropion were regularly assessed in the clinical treatment setting.
RESULTS
Ten patients who agreed to receive bupropion treatment were recruited in this study. Table 1 shows the demographics, clinical characteristics, virological response, and the efficacies and adverse effects of bupropion treatment. After bupropion therapy for 8 weeks, 4 (of 10) patients met the criteria for remission (total scores less than or equal to 7), and 5 patients met the criteria for response (at least 50% reduction in total scores). Four of the 10 patients had a 50% decrease in the CFQ for fatigue symptoms, and 5 patients had 50% decrease in NRS for neuropsychiatric symptoms. Figure 1 illustrates the rates of responders and remitters at visits of weeks 0, 4, and 8.
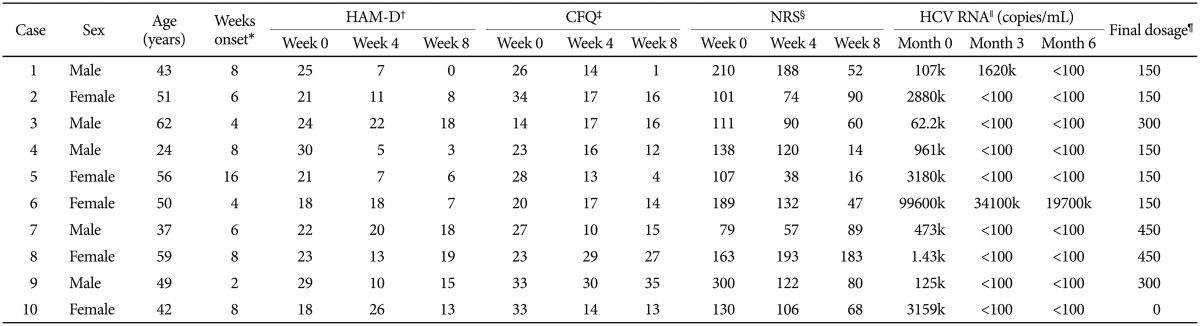
Demographic, psychometric and medical information for the 10 hepatitis C viral infected patients receiving bupropion therapy for interferon-alpha-induced depression
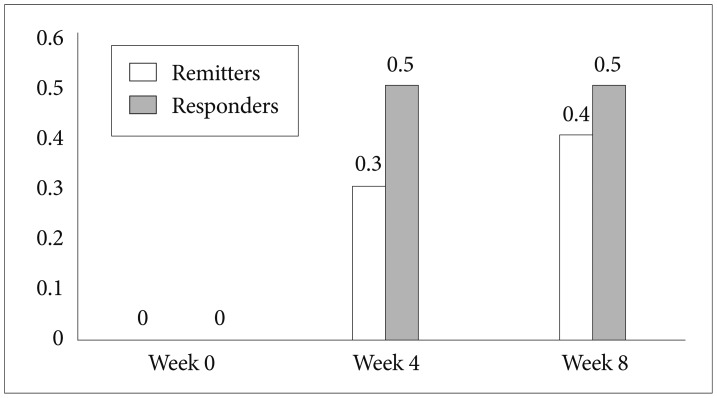
The rates of response and remission in 10 hepatitis C viral infected patients receiving bupropion therapy for interferon-alpha-induced depression at visits of weeks 0, 4, and 8.
The final total daily bupropion dose was between 150 mg and 450 mg. Headache (n=4), GI upset (n=3), and poor appetite (n=3) were among the most common adverse events after bupropion therapy. One patient was withdrawn from bupropion treatment because he developed a seizure attack when given 300 mg of bupropion per day.
DISCUSSION
The main finding is that bupropion treatment reduced somatic and depressive symptoms induced by IFN-alpha. To our knowledge, this is the first study to demonstrate that NDRI is effective in IFN-alpha-induced depression. There have been a few studies about the effects of SSRIs on IFN-alpha-induced depression.3,4 However, symptoms of depression, anxiety, cognitive dysfunction and pain seem to more be more responsive, whereas symptoms of fatigue and anorexia are less responsive.3 These data demonstrated distinct phenomenology and treatment responsiveness of symptom dimensions induced by IFN-alpha, and suggests that different mechanisms mediate the various behavioral manifestations of cytokine-induced sickness behavior.3 The statistic power was 99% at the alpha level of 0.05 with a sample of 10 subjects with the HAMD difference of mean and SD from baseline the endpoint was 12.4 and 8.3, respectively. The power analysis indicates that the treatment effect might be robust to detect the difference of approximately 10 points in HAMD, and we we will need 12 subjects per group (bupropion vs. placebo) to conduct a double-blind, placebo-controlled clinical trial in future investigation.
Bupropion has several specific pharmacological characteristics as compared to SSRI. Firstly, A number of studies suggest that norepinephrine (NE) and dopamine (DA) play a key role in the pathophysiology of sleepiness, and fatigue.7,8,9 By enhancing NE and DA both in cortex and in subcortical areas, bupropion might has a better clinical profile in improving fatigue and sleepiness.7,8,9 Secondly, bupropion is a selective NE and DA reuptake inhibitor that is devoid of several serotonergic-associated side effects, such as sexual dysfunction, weight gain, and sedation.7 Thirdly, the inflammatory mechanism plays a key role in the pathogenesis of depression, especially in somatic symptoms of depression.2 The newly found anti-inflammatory characteristics of bupropion might be a mechanism for the effect of bupropion in IFN-alpha-induced somatic symptoms.10 Finally, SSRI-related adverse effects, including gastrointestinal bleeding, retinal haemorrhaging, and hepatotoxicity, are of specific consideration in HCV patients with risks of liver decompensation.6 However, whether bupropion is associated with less occurrence of above-mentioned risk would need more clinical investigations.
Patients receiving bupropion for IFN-alpha-induced depression showed a virological response rate of approximately 90% in our study. Interestingly, the overall virological response rate is around 60% in patients receiving IFN-alpha for hepatitis C infection. Prior studies found that patients who became depressed while on IFN-alpha and ribavirin treatment and started on SSRIs achieved higher SVR rates compared with placebo,11 implying that antidepressant medications may have a direct effect on immunity. However, the relationship among virological response, IFN-alpha-induced depression, and the use of bupropion or other antidepressants in patients with hepatitis C infection would need further research.
One patient developed a seizure attack during bupropion treatment. Bupropion exposure was associated with a higher incidence rate of seizure, ranged from 0.35% to 0.44% at doses up to 450 mg/day, as compared with to the incidence of 0.07% to 0.09% with exposure to other newer antidepressant.7 In addition, seizures during IFN-alpha therapy have been reported in approximately 0.16-1% of patients,12 although the mechanism underlying IFN-alpha-induced seizures is unknown. It needs more careful observation and studies to know whether the concomitant use of bupropion and IFN-alpha would increase the risk of seizure attack.
The study is limited by its small sample size and the absence of a placebo-control group. In the future, double-blind controlled trials involving a comparison with placebo, SSRIs, or other interventional or preventive strategies,13 with close monitoring viral clearance rate and electroencephalograph examinations would be warranted.
Acknowledgments
The work was supported by the following grants: NSC101-2628-B-039-001-MY3 and NSC 103-2923-B-039-002-MY3 from the National Science Council; NHRI-EX101-10144NI from the National Health Research Institute; and DMR-101-081, DMR-102-068 and DMR-103-078 from the China Medical University in Taiwan.