Regional Brain Volume Changes in Catholic Nuns: A Cross-Sectional Study Using Deep Learning-Based Brain MRI Segmentation
Article information
Abstract
Objective
Religious behaviors are considered as complex brain-based phenomena that may be associated with structural brain change. To identify the pattern of regional brain volume change in nuns, we investigated structural alterations in the brains of nuns using a fast processing automated segmentation method based on deep learning algorithms.
Methods
We retrospectively reviewed the medical records of the catholic sisters between the ages of 31 and 80 who are members of the charity of St. Vincent de Paul of Korea. A total of 193 asymptomatic subjects (86 nuns and 107 control subjects) received comprehensive health screening and underwent brain MRI scans. We compared cortical and sub-cortical volume between groups across multiple locations using our in-house U-Net++ deep learning-based automatic segmentation tool.
Results
Compared to the control group, the nun group displayed increased gray matter volume in the right lingual cortex, left isthmus-cingulate, posterior-cingulate, rostral-middle-frontal, superior-frontal, supramarginal, temporal-pole cortices, and bilateral pars-triangularis cortices after correction for multiple comparisons. On the other hand, the nun group showed reduced gray matter volume in the temporal and parietal regions relative to healthy controls.
Conclusion
Our study suggests that spiritual practice may affect brain structure, especially in several frontal regions involved in a higher level of insight function.
INTRODUCTION
Religiosity has an important role in health promotion and disease prevention, and many studies have provided evidence for the protective effect of religiosity on physical and mental health [1-6]. In particular, epidemiological studies have provided evidence for an inverse relationship between religiosity and psychiatric disorders; it was reported that the risk of mental disorders such as depression [7] and dementia [8,9] was lower among nuns compared to the general population. Accordingly, religious belief and behavior are considered as complex brain-based phenomena that may be associated with functional and structural brain change. However, there is limited evidence on neurological mechanisms that could explain their relationship, and the mechanisms by which religion affects health status are still unknown.
The recent advance in neuroimaging techniques can help investigators to non-invasively analyze brain structure and function in various conditions. Thus, neuroimaging study may provide a possible explanation for the exact neurological mechanism of religiosity in health. Earlier studies using magnetic resonance imaging (MRI) have shown that religiosity is associated with alterations in brain structure and function in the general population [10-12]. Several studies have reported that individuals with a higher belief in religion or spirituality had a higher frontal cortical thickness [11,13] and decreased default mode network connectivity [14]. In contrast, other studies found greater atrophy in the hippocampus [15] and decreased temporal lobe blood flow [16] in specific religious groups. This conflicting result might be due to the difficulty of modeling clinical studies of religious intervention.
Most of the previous studies have focused on the acute effects of religious practices, and few studies have evaluated the long-term effects of religion on the differences in brain structure or function. It is not easy to accurately measure religious activity, and there is a subtle difference between participation in religious services and religiosity. Given the uniform religious life of the clergy, studies on clergy such as nuns and priests can overcome these limitations. However, no neuroimaging studies have been reported in nuns compared to the general population so far. A few studies on neurological changes in nuns had been limited to a simple head size measurement [17] or post-mortem brain biopsies [18,19]. Considering head circumference is an unreliable measure of brain volume, more accurate analysis using magnetic resonance scans may lead to stronger associations between religiosity and neuroanatomy.
The present study aimed to identify the pattern of regional brain volume change in nuns. Thus, we investigated structural alterations in the brains of nuns using a fast processing automated segmentation method based on deep learning algorithms.
METHODS
Subjects
This study was conducted by retrospectively checking the medical records of the catholic sisters between the ages of 31 and 80 who are members of the charity of St. Vincent de Paul of Suwon, Korea. The control group was recruited from noncleric females who underwent a health check-up during the same period. All subjects received comprehensive health screening and underwent brain MRI scans at the health promotion center of St. Vincent’s Hospital in South Korea between January 2010 and December 2019. General health check-ups were provided biennially for all subjects by the Korean National Health Insurance Service (NHIS), and additional periodic health examinations were performed at the hospital affiliated with the convent. All subjects were excluded if they had concurrent or a history of diseases that could affect brain structure or function, such as stroke, traumatic brain injury, brain tumor, neurodegenerative disorders, anxiety, and depression, or if they were taking or had previously taken psychotropic medications that could affect the nervous system. Those who with estrogen replacement therapy in the postmenopausal state were also excluded. To rule out cognitive impairment, the Korean Dementia Screening Questionnaire-Cognition (KDSQ-C) was applied to individuals aged 66 yr. In addition, participants with a history of all-cause dementia (International Classification of Disease, 10th Revision [ICD-10] codes: F00, F01, F02, F03, G23.1, G30, G31) during the follow-up period from 2010 to 2022 were excluded.
After excluding subjects with missing or duplicate values in the observation result that was required for the study, a total of 193 asymptomatic subjects (86 nuns and 107 normal control subjects) were selected for the study. This study was approved by the Research Ethics Committee of the College of Medicine, The Catholic University of Korea (IRB Number: XC20RIDI0058, approved May 2020).
Brain imaging and data processing
MR image acquisition
T1-weighted optimized high-resolution 3D magnetizationprepared rapid acquisitions of gradient echo (3D-MPRAGE) images of the brain were acquired using a 3T scanner equipped with a 32-channel head coil (Verio; Siemens, Erlangen, Germany). Image acquisition parameters were as follows: number of slices, 160; voxel resolution, 0.49×0.49 mm2; thickness, 1 mm; flip angle (FA), 9°; field of view (FOV), 250×250 mm2; repetition time (TR), 1,900 ms; inversion time (TI), 900 ms; and echo time (TE), 2.46 ms.
Brain MRI segmentation
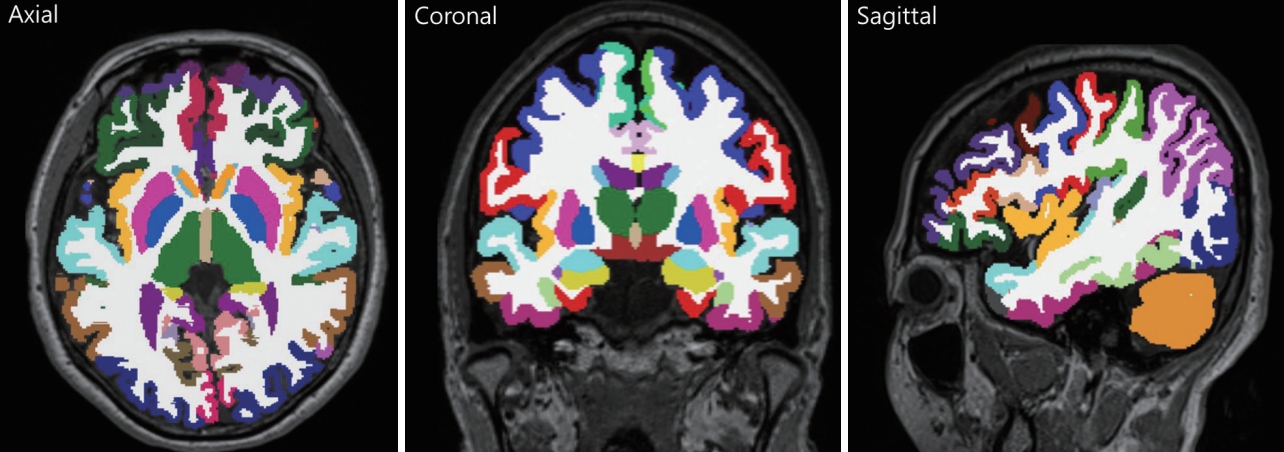
This study utilized our in-house deep learning-based automatic segmentation tool. T1-weighted MRIs were preprocessed by the U-Net++ deep learning-based segmentation processing [20]. We used our deep learning method for extracting numerical data into a table format. The set of 97 layer subvolume-based features used for the training procedure are described in Supplementary Table 1 (in the online-only Data Supplement). Figure 1 shows the segmentation result of brain sub-volumes. The detailed algorithms for our deep learningbased segmentation methods are described in the Supplemental Material (in the online-only Data Supplement).
Statistical analysis
The data were analyzed using the Statistical Package for the Social Sciences version 21 (IBM Co., Armonk, NY, USA) and were presented as means±standard deviation. Independent t-tests were used to compare characteristics at baseline between the nun and control groups. A two-tailed p-value<0.05 was considered statistically significant. In addition, to assess between-group differences in structural measures, the volumes of the 97 automatically segmented brain regions were imported into the SPSS software for statistical analyses, and analysis of covariance (ANCOVA) was performed in a general linear model of SPSS. Group variables (nun vs. control group) were included as independent variables, with the extracted cortical and subcortical volume (in cm3) as dependent variables. We included age, educational level, and total intracranial volume (TICV) as covariates to prevent potential confounding effects. For multiple comparisons, Bonferroni corrections were applied to minimize the type I error risk by dividing our p-value by the number of segmented areas: p<0.05/97 (number of comparisons in bilateral hemispheres)=5.15×10-4.
RESULTS
Baseline characteristics of the study participants
Table 1 summarizes the baseline characteristics of the study participants. Although there was no significant difference in education level between the two groups, participants in the nun group were older than those in the control group (61.41±8.48 years vs. 54.56±10.86 years, p<0.001). Body mass index (26.97±4.51 kg/m2 vs. 23.36±3.47 kg/m2, p<0.001) and waist circumference (91.60±10.46 cm vs. 79.37±9.74 cm, p<0.001) were significantly higher in the control group than in the nun group. The TICV was also greater in the control group than in the nun group (1,394.95±92.49 cm3 vs. 1,302.61±235.1 cm3, p<0.001). The glucose, lipid profile, alanine aminotransferase (ALT), and gamma-glutamyl transferase (γ-GTP) were not significantly different between groups. However, the systolic and diastolic blood pressure was lower in the nun group than in the control group (p<0.05).
Regional brain volume differences between the nun and control groups
For the cortical and subcortical volume analyses, the ANCOVA, adjusted for age, education, and TICV revealed a significant volume increase in the left isthmus-cingulate, pars-triangularis, posterior-cingulate, rostral-middle-frontal, superiorfrontal, supramarginal, temporal-pole, and right lingual, pars-triangularis cortices in the nun group compared with the control group after correction for multiple comparisons (p<0.0005, Figure 2A and Table 2). The volume of the cerebellum-white-matter, corpus callosum, and right thalamus was also higher in the nun group compared to the control group (p<0.0005).
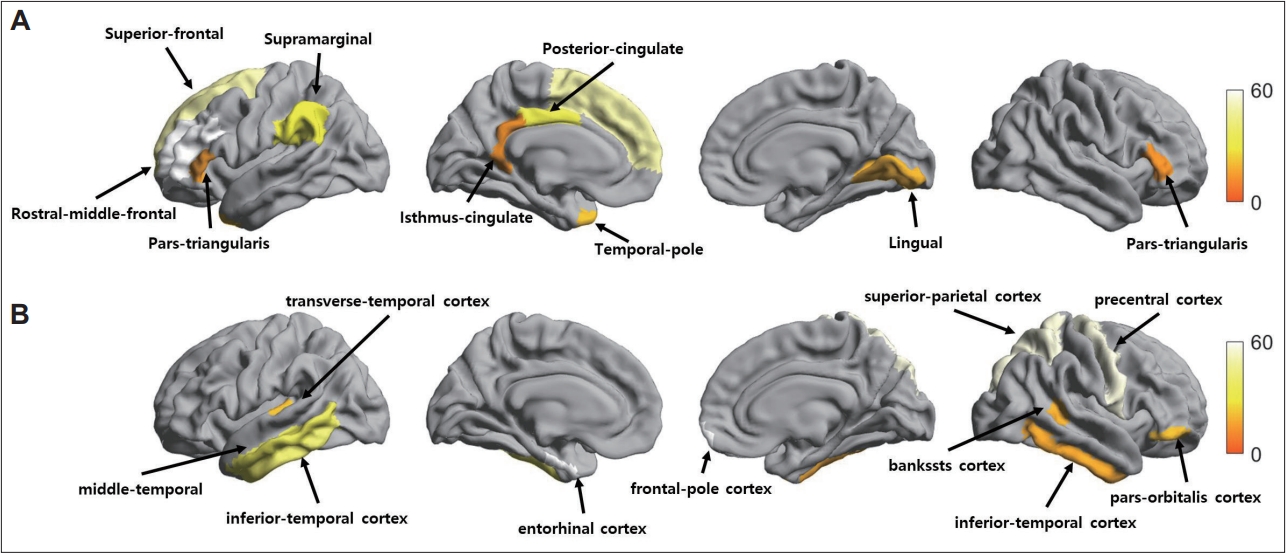
Regional brain volume differences between the nun and control groups. A: Statistical maps showing a significant volume increase in the left isthmus-cingulate, pars-triangularis, posterior-cingulate, rostral-middle-frontal, superior-frontal, supramarginal, temporal-pole, and right lingual, pars-triangularis cortices in the nun group compared with the control group. B: A cortical volume reduction in the nun group relative to the control group in the left entorhinal, inferior-temporal, middle-temporal, transverse-temporal, and right bankssts, frontal-pole, inferior-temporal, pars-orbitalis, precentral, superior-parietal cortices. Statistical significance was tested using analysis of covariance adjusted for age, education, and total intracranial volume. Regions that remained significant after correction for multiple comparisons are displayed (p<0.0005, Bonferroni correction).

Regional brain volume where a significant atrophy was observed in the control group compared to nuns
On the other hand, a group comparison analysis of regional cortical volume between the nun and control groups revealed a significant reduction in the cortical volume of the nun group in the left entorhinal, inferior-temporal, middle-temporal, transverse-temporal cortices, and right bankssts, frontal-pole, inferior-temporal, pars-orbitalis, precentral, superior-parietal cortices compared with those of the control group (p<0.0005; Figure 2B and Table 3). For the subcortical structures, the nun group showed a significant reduction in the left accumbens, caudate, putamen, and right caudate, pallidum, putamen compared to the control group (p<0.0005).
DISCUSSION
In the present study, we investigated regional brain volume differences between the nun and control groups using a deep learning algorithm. We found group differences in several brain regions; compared to the control group, the nun group displayed increased gray matter volume in the right lingual cortex, left isthmus-cingulate, posterior-cingulate, rostral-middle-frontal, superior-frontal, supramarginal, temporal-pole cortices and bilateral pars-triangularis cortices. On the other hand, the nun group showed reduced gray matter volume in the temporal, and parietal regions relative to healthy controls. To the best of our knowledge, this is the first study evaluating the brain structural changes in nuns using MRI.
Although religious activity appears to improve physical and mental health, the exact neurological mechanisms by which religiosity affects health are uncertain. Identifying differences in regional brain volume between nuns and controls might explain the role of religion in brain structural changes. However, few studies had examined the relationship between religiosity and structural neuroanatomy so far. A small number of studies have found that religious activity is correlated with the volume of specific brain regions, but the focus has been limited to epilepsy patients [21] and short-term religious practices [22]. The current study extends these researches by examining whole-brain segmentation in nuns. The result of our study might help to understand the relationship between brain structure and religion.
In this study, the nun group exhibited significantly increased cortical volume for a number of frontal regions, namely the left rostral-middle-frontal, superior-frontal, and bilateral parstriangularis cortices. Of these regions, the dorsolateral prefrontal cortex (DLPFC), corresponding to the rostral middle frontal region, plays a key role in the processing of cognition [23] and emotions [24]. Thus, the DLPFC is associated with many neuropsychiatric diseases, and the hypertrophy of this region might explain the low prevalence of dementia [8,9] and mood disorders [7] in the nuns. Furthermore, the DLPFC plays a crucial role in a higher level of insight functions such as executive functioning, concept flexibility, and self-monitoring [25]. Interestingly, a previous fMRI study investigating brain activity engaged during self-reflective introspection showed activated regions in the superior frontal gyrus as well as bilateral activation of the inferior frontal gyrus, which includes the pars triangularis. This is in keeping with the findings of our study and supports the hypothesis that the increased frontal lobe volume of nuns in this study might be the result of lifelong introspection and self-reflection in the nuns. In fact, previous studies showed that reduced frontal lobe volume caused a failure of metacognition [26] and a lack of insight in neuropsychiatric disorders [27]. A combination of structural, functional imaging data and clinical information gathered longitudinally will be required to understand the mechanisms by which the frontal lobe hypertrophy in nuns influences the neuropsychiatric outcome.
The control group showed smaller cortical volume in frontal regions, as well as less cerebellar white matter volume, when compared to nuns. Considering that the frontal regions found in this study (i.e., rostral-middle-frontal, superior-frontal, and pars-triangularis) are important brain regions for executive control, including inhibitory functioning, attention, impulsivity, and self-regulation [28], these structural brain differences might explain less substance abuse and better self-control in nuns. Consistent with our findings, Squeglia et al. [29] had reported volume reduction of the frontal cortex and cerebellar white matter in adolescent heavy drinkers.
The pars triangularis (BA45), which is part of Broca’s area is a speech and language-related area, and is known to be an overlapping region for semantic and syntactic functions [30]. In particular, the pars triangularis has been shown to be involved in understanding God’s intent and resolving the negative emotional significance of his lack of involvement. The hypertrophy of this area in the present study might be due to the religious lifestyle of the nuns, who spend a lot of time reading the Bible and praying. Another possible explanation is the prosocial characteristics of nuns. In general, nuns try to overcome various social prejudices and stereotyping through pro-social dialogue with heterogeneous subjects.
In this study, the left DLPFC as well as pars triangularis displayed increased cortical volume in the nun group compared with controls. Experimental evidence shows that prosocial choices positively relate to local gray matter volume and the activation of brain areas that control selfish impulsive drives, such as the DLPFC [31]. Thus, human prosociality is a consequence of cognitive control of selfish impulses. Pro-social communications between heterogeneous dyads are also relevant to recently proposed coding models relating the theory of mind with predictive processes [32]. The theory of mind refers to the capacity to understand other people by ascribing mental states to them. Possessing a functional theory of mind is considered crucial for success in everyday human social interactions and is used when analyzing, judging, and inferring others’ behaviors. The increased brain regions in the nuns in this study (i.e., DLPFC and pars-triangularis) overlapped with areas commonly activated in the theory of mind. The results of our study explain the altruistic and prosocial tendencies of the nuns. Similar to our findings, Descorbeth et al. [33] reported that left DLPFC and pars triangularis were more active during speech dialogue in high than in low-disparity groups.
Our results showed significant decreases in gray matter volume located within the temporal brain region. To date, a few neurotheological studies have reported the functional and structural brain changes according to religious and spiritual performance [16,34]. Previous data have shown that the temporal lobe, which is involved in the activation of associative and spatial orientations, is inactivated during religious experiences, whereas the area of attention is activated, particularly around the prefrontal cortex [35]. Newberg et al. [16] studied changes in blood flow rate in the brain during prayers of three Franciscan nuns. In this study, the authors found a lack of self-awareness during prayer due to decreased temporal lobe blood flow. Other researchers have also cited the temporal lobe as a potential site for mystical experiences and religious feelings [36,37]. In line with these findings, the results of our study showed the increased frontal gray matter volume and reduced temporal cortical volume in nuns compared to the control group. Together with the previous findings, our present results may indicate the greater difficulty and procedural demands for imagining and processing the intent of supernatural agents in non-clerics. Consistent with our findings, Owen et al. [15] reported hippocampal atrophy in participants reporting a life-changing religious experience, particularly among Catholics. Kapogiannis et al. [22] also reported that the temporal to occipital pathway was more active in non-religious compared to religious subjects, suggesting a lack of religious beliefs related to supernatural agents or God. Another possible explanation for temporal atrophy in nun groups might be related to stress. Those who struggle with their beliefs tend to experience higher levels of stress. From a psychoneuroendocrine perspective, this might cause a release of stress hormones that are known to reduce the regional brain volume over time. However, limited medical record data may not accurately reflect patient’s future risk of dementia; although we examined changes in mood and cognition at health screenings annually, follow-up was relatively short, considering the time for dementia occurrence. Furthermore, considering causes of dementia are multifactorial and heterogeneous, the current study did not include some risk factors of dementia, such as various genetic (i.e., apolipoprotein E ε4 allele) and familial factors. However, periodic health examinations were continuously implemented for all participants, and thus, minimized the possibility of misclassification. Further prospective studies with longer follow-up periods are required to strengthen the evidence for this conclusion and to determine the underlying mechanism of association between religiosity and brain structure.
The positive effects of religion on health were known as psychological optimism, healthy lifestyles such as low rates of smoking and alcohol drinking [38], sufficient social networks through religious activity, and reduced exposure to risky sexual behavior [39]. However, the mechanisms by which religiosity influences brain structure are unknown. One possible mechanism of the cortical changes in nuns is the altered activity of brain-derived neurotrophic factor (BDNF) by spiritual practices. A previous study reported that depressed patients with high religiosity had significantly higher serum BDNF than do low religiosity [40]. BDNF affects synaptic plasticity and dendritic and neuronal fiber growth, promotes neuronal survival, and has been considered a biological marker of brain neuroplasticity [41,42]. Indeed, it was reported that a higher religiosity or spirituality was associated with greater cortical thickness [11]. Moreover, another study had shown that the BDNF level was inversely correlated with cortical atrophy in depressed individuals [43]. Thus, religiosity might affect brain structure in catholic nuns by increasing cortical neuroplasticity and neuroprotection through BDNF. However, we did not measure BDNF in this study; thus, we could not identify the potential mechanisms through which BDNF affects brain plasticity.
There are some limitations in this study. First, we performed a cross-sectional study of healthy subjects without psychiatric problems, and we could not determine the causality between structural brain changes and mental disorders. Second, given the small sample size in this preliminary study, the generalizability of our findings should be approached with caution. However, our study design has strength in allowing for minimizing potential confounding factors. A wide variety of factors that confound most epidemiologic studies were eliminated or minimized in the present study because of the relative homogeneity of the sisters’ environments and lifestyles. Participants in this study had the same reproductive and marital histories; had same religious backgrounds; had similar social networks, activities, and support; did not smoke or consume excessive alcohol; had similar education level, occupations, income, and socioeconomic status; all lived in the same houses; ate food prepared in the same kitchens; and had comparable access to similar preventive and medical care services. Moreover, we analyzed changes in brain structure and intrinsic religious beliefs, which was different from earlier studies in which only attendance at religious services and religious denominations were investigated. It was known that intrinsic religious beliefs, rather than externalized components of religiosity, were associated with psychiatric disease [11]. Thus, the results of our study provide robust evidence for the association between religiosity and neuroanatomy. Considering brain atrophy may be associated with many neuropsychiatric disorders, further prospective studies are required to evaluate the relationship between brain structural changes in nuns and clinical outcomes over time.
In conclusion, our study provides a unique model to explain the role of religion in brain structure. The results of our study suggest that spiritual practice may affect cortical and subcortical brain volume, especially in a number of frontal regions involved in a higher level of insight function. Although these results require further study, locating changes in brain regions related to spiritual practice may provide researchers with another clue into how spirituality and religiosity contribute to mental health benefits. The results of the present study should be considered preliminary, and further studies are needed to reveal that the changes in specific brain areas in nuns may serve as a compensatory or protective mechanism against certain psychiatric diseases. Nevertheless, the unique characteristics of our study, which minimizes potential confounding factors, strengthen the relationship between religiosity and brain structural change. The combination of brain imaging and clinical information collected longitudinally will be required to understand the dynamic mechanisms by which the structural brain changes in nuns influences health outcome.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.30773/pi.2022.0165.
Summary of brain sub-volumes
U-Net++ deep learning architecture
Three-dimensional patch-based training scheme explanation.
Deep learning-based segmentation result.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Se-Hong Kim, Youngmi Eun, Min-Kyun Im, Donghyeon Kim. Data curation: Se Jin Park, Sun Myeong Ock, Se-Hong Kim, Youngmi Eun, Bo-Kyung Kim, Tae-Hong Kim. Formal analysis: Youngmi Eun, Ju-Hye Chung. Funding acquisition: Donghyeon Kim, Ju-Hye Chung, Se-Hong Kim, Tae-Hong Kim. Investigation: Bo-Kyung Kim, Ju-Hye Chung, Se Jin Park. Methodology: Ju-Hye Chung, Donghyeon Kim. Project administration: Donghyeon Kim, Se-Hong Kim. Resources: Donghyeon Kim, Se-Hong Kim. Software: Ju-Hye Chung. Supervision: Donghyeon Kim, Se-Hong Kim, Sun Myeong Ock. Validation: Ju-Hye Chung. Visualization: Ju-Hye Chung. Writing—original draft: Youngmi Eun, Ju-Hye Chung, Donghyeon Kim, Se-Hong Kim. Writing—review & editing: Youngmi Eun, Ju-Hye Chung, Sun Myeong Ock, Donghyeon Kim, Se-Hong Kim.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1F1A1062937 and 2020R1G1A1013599).