Old Age, Female Sex, and Psychotropic Medications Predict Hyposalivation But Not Heart Rate Variability
Article information
Abstract
Objective
To explore risk factors for dry mouth and examine the clinical utility of the heart rate variability (HRV) test in the prediction of dry mouth.
Methods
Every outpatient, who underwent tests for both unstimulated salivary flow and HRV, was retrospectively reviewed. After excluding seven subjects, the demographics and clinical factors in 70 total patients were collected. Based on objective salivary flow rates, patients were classified into normal (≥0.2 mL/min) or hyposalivation groups (<0.2 mL/min), and inter-group comparisons were performed with a two-tailed statistical significance of 0.05.
Results
Patients with subjective dry mouth were significantly more likely to show hyposalivation. Advanced age, female sex, and current use of psychotropic medications were identified as risk factors for dry mouth. However, dry mouth was not associated with any HRV parameters.
Conclusion
HRV test did not demonstrate a clinical utility in predicting dry mouth. Because subjective dry mouth is significantly associated with objective hyposalivation, a simple probing question would be useful for early recognition of dry mouth. Clinical attention is required for patients meeting criteria of older age, female, and/or using psychotropic prescriptions. Prompt management of hyposalivation may improve quality of life and clinical outcome by enhanced treatment adherence.
INTRODUCTION
Xerostomia (dry mouth) is a frequently encountered symptom in clinical practice with an estimated pooled prevalence of 22% in general population according to a meta-analysis [1]. Dry mouth is even more prevalent among patients with mental illness, with up to 41%–76% of bipolar disorder; 35%–47% of major depressive disorder; and 39%–69% of schizophrenia patients reporting having suffered from dry mouth [1-5].
Underlying mechanisms for the increased prevalence of dry mouth among psychiatric patients may result from anticholinergic action of psychotropic prescriptions and/or psychological symptoms per se insofar as anxiety has been reported to be a risk factor for dry mouth [4,6]. Despite such high prevalence, dry mouth symptoms are often overlooked in comparison to other psychiatric symptoms in patients with mental illness. Indeed, the clinical attention of mental health professionals is more focused on mental health than oral health. Nevertheless, the negative consequences of hyposalivation may extend beyond oral health to exertion of detrimental effects on both general and mental health in affected individuals.
Hyposalivation, or salivary gland hypofunction, is the condition of having reduced saliva production. Saliva serves multiple functions including as a lubricant, pH buffer, and antimicrobial defense [7]. Therefore, patients with dry mouth may experience problems in speech, eating, and/or upper gastrointestinal symptoms. Reduced anti-microbial defense capabilities due to hyposalivation may increase dental caries (tooth decay), periodontal disease, halitosis (bad breath), and opportunistic infections such as Candida albicans [7]. Furthermore, hyposalivation has recently been suggested as a potential risk factor for respiratory infections such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [8]. At the extreme end of potential complications, polydipsia-induced severe hyponatremia is a potentially life-threatening consequence of dry mouth [9,10].
In addition to the general health risks, dry mouth-induced impairments in speech, swallowing, and halitosis may cause significant psychological distress and reduce the quality of life in those that suffer [11]. Furthermore, the condition may worsen mental health by increasing the relapse risk due to treatment non-adherence. Medication side effects have been repeatedly reported as a major underlying cause of psychotropic non-adherence [3,12]. A meta-analysis recently revealed that only 71.1% of patients are adherent to oral antipsychotics [13]. To improve quality of life and clinical outcomes in patients by reducing treatment non-adherence, dry mouth must not be overlooked and the risk factors should be assessed for its early recognition.
It remains unclear which factor—the usage of psychotropic medications or psychological symptoms—plays a more determinant role in the development of dry mouth in patients with psychiatric disorders. While psychotropics with high anticholinergic properties increase the risk of hyposalivation, a sense of oral dryness may also occur as a physiologic reaction to stress. The parasympathetic autonomic nervous system (PNAS) induces saliva secretion by cholinergic activity [14]. The PNAS is counteracted by the stress-responsive sympathetic autonomic nervous system.
Appropriate identification and understanding of the respective contributions of psychotropics or psychological stress to hyposalivation has a great deal of clinical significance. For instance, mistaking psychotropic-induced dry mouth as anxiety or another stress-related symptoms may lead to failure in addressing the condition as a side effect of medication, thereby increasing the risk for non-adherence risk. In contrast, when dry mouth due to increased sympathetic tone is perceived as an anticholinergic side effect of psychotropic medication, such misperception may lead to under-treatment and ultimately increase the illness burden through residual symptoms. Therefore, determining the relative contributions of psychological stress versus anticholinergic side effects of dry mouth will greatly aid clinical decision-making.
This study aims to compare objective anxiety measurements against tests for heart rate variability (HRV), a widely used surrogate marker of the autonomic nervous system, in order to investigate their influence on dry mouth in patients with overlapping symptoms.
Dry mouth is influenced by demographic factors such as age and other clinical factors such as the usage of certain medications or the presence of chronic illnesses including hypertension [15,16]. Accordingly,, there is a strong need to comprehensively investigate the relative contribution of the aforementioned biological and psychological factors in patients with psychiatric disorders.
This study objectively measures salivation and explores potential risk factors in predicting hyposalivation in psychiatric outpatients. Such knowledge will enable early and more accurate identification of the population at risk. It may also elucidate ways for better management of dry mouth symptoms, leading to improved quality of life and clinical outcomes through greater treatment adherence.
METHODS
Subjects
A retrospective review was performed for every new outpatient who underwent both unstimulated salivary flow and HRV tests in the department of psychiatry of Eunpyeong St. Mary’s Hospital from May 2019 to October 2020. All subjects’ medical records were reviewed and utilized, with the exception of those that fit exclusion criteria. Exclusion criteria were 1) missing or declined anxiety measurements and 2) comorbid medico-surgical conditions affecting saliva secretion, such as the presence of Sjogren’s syndrome and/or cancer treatment history with radiation or chemotherapy. Of 77 patients with saliva flow and HRV tests, 70 were included in our final analysis after excluding seven patients for missing data (n=5) and comorbid medico-surgical conditions (n=2). This study was conducted in accordance with the Declaration of Helsinki and was reviewed and approved by the Institutional Review Boards of Eunpyeong St. Mary’s Hospital at The Catholic University of Korea (PC21RASI0069).
Measurements
In addition to saliva flow rate and HRV measurements, clinical information was collected including past medical diagnoses, psychotropic prescriptions, psychiatric diagnoses, and subjective dryness. For a psychological measure of anxiety, total Beck Anxiety Inventory (BAI) scores were collected.
Subjective dryness
Before tests were conducted to measure saliva flow, patients responded “yes” or “no” to a question asking whether they had dry mouth symptoms.
Unstimulated whole saliva flow rate (UWSFR)
Saliva collection was performed with patients in an upright sitting position. Cotton balls were placed inside the mouth—with positioning both over and under the tongue and between patients’ teeth and chin bilaterally. After five minutes to allow saturation of the cotton balls by saliva, the net weight of the cotton balls was recorded. Patients’ UWSFR was measured via a gravitation method with the presumption of 1 g of saliva being equivalent to 1 mL, using a laboratory scale with an accuracy of 0.01 g and repeatability of ±0.005 g (MW-IIN High Precision Micro Weighing, CAS, Seoul, Korea).
BAI
The BAI measures the severity of anxiety symptoms on a four-point Likert scale over the preceding week. It is a widely used 21-item self-measured inventory, which has been previously validated in the Korean language [17].
HRV
HRV was recoreded with three channel electrocardiograms (ECG) leads. The ECG electrodes were placed at both wrists and the left ankle of each subject. After subjects were given about three minutes of rest to adapt to the experiment conditions, the HRV tests were performed for five minutes with subjects in the seated position at complete rest using a WISE-8000 HRV analyzer (MooYoo Instrument Co., Ltd, Seongnam, Republic of Korea). The amplified ECG signals were detected at 500 Hz, and they were digitized.
The following HRV parameters were computed using frequency-domain spectral analysis: very-low-frequency power (range, ≤0.04 Hz), low-frequency power (LF; 0.04–0.15 Hz), high-frequency power (HF; 0.15–0.4 Hz), and the ratio of low frequency to high frequency (LF/HF ratio). While the LF band is known to reflect the sympathetic nervous system (SNS), the HF band is considered to reflect parasympathetic nervous system (PNS) modulation. The LF/HF ratio has been previously suggested to reflect the balance between SNS and PNS activity [18,19].
The square root of the mean squared differences of successive normal-to-normal intervals, which is measured in ms, was collected as a time-domain measurement. This root mean square of successive differences (RMSSD) between normal heartbeats is known to reflect PNS activity [20].
Statistical Analyses
To examine risk factors that contribute to hyposalivation, subjects were classified into two groups using objectively measured salivary flow rates. Based on salivary flow rate per minute, patients were classified as normal (≥0.2 mL/min, n=32) or as having hyposalivation (<0.2 mL/min, n=38) in accordance with the literature [21]. Inter-group comparisons were conducted using χ2 or Fisher’s exact tests for categorical variables and t-tests for continuous variables. To explore the relationships between objective salivary flow rates and xerostomia risk factors, correlation analysis was conducted to calculate Kendall’s tau-b coefficients. Analyses were done using statistical software SPSS Statistics for Windows, version 19 (IBM Corp., Armonk, NY, USA) with a two-tailed statistical significance of 0.05.
RESULTS
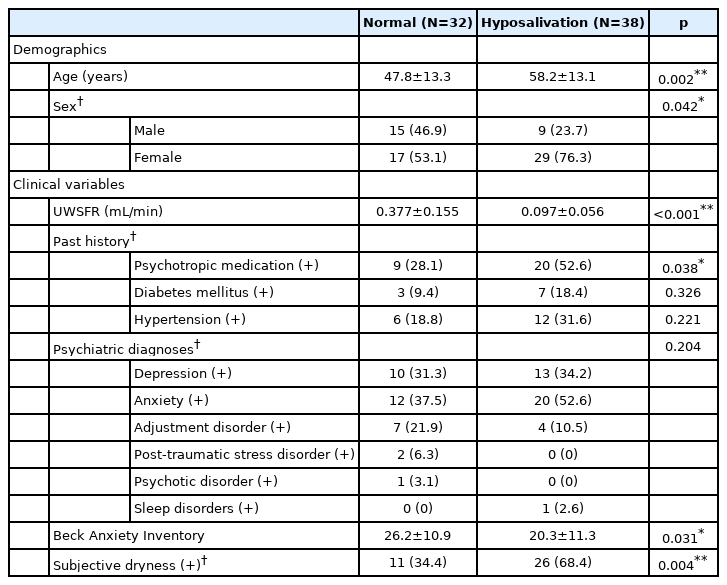
The mean UWSFR was significantly lower in the hyposalivation group (0.10 mL/min) in comparison to the group with normal UWSFR (0.38 mL/min, p<0.001). In terms of demographics, patients in the hyposalivation group were significantly older (58.2 years old vs. 47.8 years old, p=0.002) and more likely to be female (76.3% vs. 53.1%, p=0.042) (Table 1).
With regard to the clinical factors, patients on psychotropic medications demonstrated a higher association with low UWSFR than psychotropic-naïve patients (52.6% vs. 28.1%, p=0.038). Patients with subjective complaints of dry mouth were more likely to be confirmed for hyposalivation with objective UWSFR measures (68.4% vs. 34.4%, p=0.004) than patients without subjective complaints of xerostomia (Table 1).
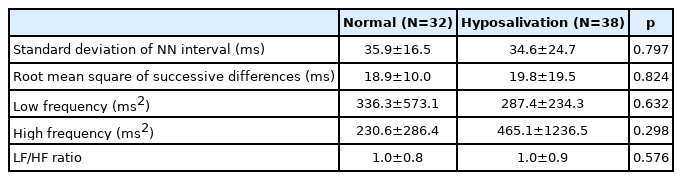
Total BAI scores, however, were significantly lower in the hyposalivation group than in the normal group (20.3 vs. 26.2, p=0.031). In addition, the hyposalivation group did not demonstrate any significant differences in comparison to the group with normal salivary flow in HRV parameters such as HF or RMSSD (Table 2).
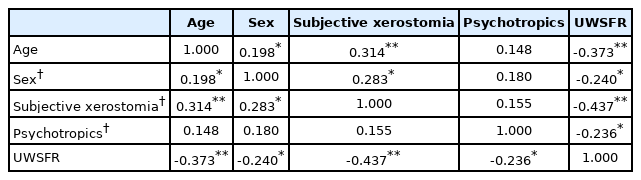
In correlation analysis, UWSFR showed significant inverse correlation with age (τ=-0.373, p<0.001). Subjective xerostomia (τ=-0.437, p<0.001), being female (τ=-0.240, p=0.017), and having a current prescription for psychotropic medication (τ=-0.236, p=0.019) also showed significant correlations with objective salivary flow (Table 3).
DISCUSSION
Saliva serves various important roles including lubrication, pH buffering, and immunologic barrier against infections [7,8]. Xerostomia significantly impairs quality of life due to difficulty in speech, halitosis, and may act as a direct underlying cause of poor oral intake (a common symptom in patients with mental disorders) due to decreased functionality in mastication, difficulty with swallowing, or dysgeusia (altered taste) [7]. However, dry mouth is often overlooked in patients presenting with mental health problems insofar as oral health is not a primary therapeutic focus among mental health professionals.
Nevertheless, dry mouth is a prevalent condition among patients with mental disorders and may worsen the clinical course unless adequately addressed. As a side effect of some psychotropic medications, xerostomia may contribute indirectly to deterioration in mental health by posing an increased risk for relapse due to treatment non-adherence. Accordingly, dry mouth should be considered more seriously in clinical practice to improve treatment outcomes.
Previous research has reported that advanced age and female sex may be demographic risk factors for dry mouth. This study also demonstrates that being older and being female are significantly associated with hyposalivation, which is in line with findings from existing studies [15,16]. While age had a strong relationship (τ=-0.373) with objective salivary test results in correlation analyses, female sex demonstrated a modest association (τ=-0.240) herein.
Age-related changes may render elderly people at greater risk of xerostomia due to a natural decline in salivary secretion. Because older people are more likely to be affected by chronic illnesses such as hypertension, diabetes mellitus, or arthritis, clinical attention should be increased in this population. Increased medical comorbidities and a greater likelihood of polypharmacy make elderly populations more prone to hyposalivation [22].
Previous research has indicated that UWSFR and salivary glands in females are significantly smaller than in males [23]. This biological difference may underlie the increased vulnerability of females to dry mouth. These higher risks suggest that medications with lower anticholinergic properties should be considered as the first-line psychopharmacologic therapy in patients that are elderly and/or female.
Nevertheless, the role of psychiatric conditions in the development of dry mouth is rather obscure in contrast to demographic factors. Depression and anxiety have been previously reported to be associated with xerostomia [4]. On the other hand, prescription of psychotropics may also contribute to development of dry mouth as an adverse effect of medication [6]. Because psychiatric symptoms per se and psychopharmacologic therapy may both act as risk factors, further understanding of their relative contribution to hyposalivation will enable better prevention and management of dry mouth in treatment processes.
Among patients herein, psychotropic medication status exerted a significant effect on dry mouth (52.6% vs. 28.1%, p=0.038). These findings are in line with existing literature [4,6]. Accordingly, polypharmacy should be minimized and pharmacodynamics must be more thoroughly considered to reduce the risk of dry mouth in elderly patients. Whether administering antidepressants or antipsychotics, first-line treatment option should be sought from medications with the lowest anticholinergic profiles. Although advanced age is associated with an increased risk of extrapyramidal symptoms [24], prophylactic anticholinergics should be avoided. Furthermore, treatment with anticholinergics should be discontinued or minimized once extrapyramidal issues are resolved. As a standard of care, dry mouth screening should be routinely performed in elderly females before and during pharmacotherapy.
Psychiatric symptoms were shown not to worsen xerostomia in this study, which contradicts findings from the literature [4]. Surprisingly, levels of anxiety were demonstrated to be lower in the hyposalivation group herein. This suggests that the contribution of psychiatric symptoms per se to dry mouth may be relatively small in comparison to the contribution of psychotropics. This highlights that awareness of treatment-emergent iatrogenic hyposalivation should be increased and the affliction more actively monitored during follow-up phases of treatment. However, the finding above may stem from the significantly higher proportion of patients reporting current usage of psychotropic medications in the hyposalivation group. Because this study was undertaken in a university hospital setting, a substantial proportion of referred patients had been prescribed psychotropics prior to their hospital visits. This may have served as a common underlying reason for reduced anxiety levels, while increasing the occurrence of dry mouth among subjects herein. To clearly distinguish the respective contributions of psychotropics versus psychiatric symptoms to the development of hyposalivation, prospective studies should be performed in drug-naïve patients.
Measures of both HF power and RMSSD may reflect PNS and are known to be lower in depression and/or anxiety disorders [20,25,26]. Accordingly, these measures were key parameters for examination in this study. Although we attempted to investigate the potential role of HRV parameters in the prediction of dry mouth, no HRV parameters were found to be statistically different between patients in the hyposalivation group and patients without hyposalivation. This finding may have resulted from the confounding effects of psychopharmacologic treatment. Two weeks of antidepressant treatment has been reported to improve reduced HF power in depressed patients [26]. A greater proportion of patients in the hyposalivation group reported receiving pharmacologic treatment, and their anxiety levels were significantly lower than the anxiety levels of patients without hyposalivation. Treatment may have stabilized HRV indices and masked its potential utility. HF power, however, has been suggested not to represent vagal tone [20]. A larger clinical trial with drug naïve patients may be able to verify the true potential of HRV parameters in predicting vagal function in patients with mental illness.
In this study, positive responses to a simple inquiry about dry mouth predicted actual hyposalivation in comparison to patients with no complaints of dry mouth (68.4% vs. 34.4%, p=0.004). Objective salivary flow measures, whether stimulated or not, are able to accurately capture patients with xerostomia. The routine application of objective salivary secretion measurements to every patient may not be feasible, however, and would certainly be time-consuming. In this study, positive responses to inquiries about subjective xerostomia demonstrated the strongest negative association with UWSFR (τ= -0.437). Insofar as simple screening for subjective dry mouth may reliably detect reduced salivary flow, such objective salivary flow measures could be more practically applied to patients with identified risks for dry mouth before administration and during treatment with psychotropics.
Still, individual thresholds for subjective oral dryness may vary. For example, patients with high levels of neuroticism may have a lower threshold and subjects with shy or apprehensive personalities may be reluctant to complain about xerostomia. In such cases, a more discreet approach could be helpful, and clinical judgements may also be required for objective salivary flow tests.
There are some limitations to this study. First, the retrospective design of this case-control study is susceptible to selection and information bias. Although we attempted to comprehensively include all potential xerostomia risk factors and the proportion of missing records was rather small, some factors may remain uncaptured in the medical records. Second, circadian rhythms have been reported to influence salivary flow [27]. Due to the retrospective design of this study, the timing of saliva collection was not controlled. Future research should be designed to consider diurnal variations in saliva secretion. Next, the BAI, which is highly subjective, was used in this study to measure anxiety levels. This may have caused patients to be more prone to sample bias than objective ratings. In future research, replication of our methods with objective assessment tools may further corroborate our findings. Although HRV parameters in this study failed to demonstrate clinical utility in predicting salivary functioning, other potential parameters reflecting vagal tone may present a link to salivation.
Despite these limitations, this study comprehensively examines risk factors for dry mouth—a prevalent condition in patients with mental disorders—with objective salivary flow tests. Advanced age, being female, and the use of psychotropic prescription are identified as risk factors for dry mouth. Psychotropic medications are found to play a key role in dry mouth in comparison to anxiety levels or the parasympathetic activities indirectly measured by tests to detect HRV. When patients present with dry mouth, rather than minimizing the symptom as a neurotic complaint, clinicians should look into a possible link to any current psychopharmacological treatment and make appropriate adjustments to reduce the risk of treatment non-adherence and aggravation of mental health conditions.
Notes
Availability of Data and Material
Datasets generated and analyzed within the study are not publicly available due to privacy restrictions but will be made available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Chang-Uk Lee, Seung-Yup Lee. Data curation: Seung- Yup Lee. Formal analysis: Seung-Yup Lee. Investigation: Min-Hyeon Park. Project administration: Seung-Yup Lee. Supervision: Kyu-In Jung, Chang- Uk Lee. Validation: Chang-Uk Lee. Writing—original draft: Seung-Yup Lee. Writing—review & editing: Kyu-In Jung, Min-Hyeon Park, Chang-Uk Lee.
Funding Statement
None