Childhood Sexual Abuse and Cortical Thinning in Adults With Major Depressive Disorder
Article information
Abstract
Objective
A growing body of evidence reports on the effect of different types of childhood abuse on the structural and functional architecture of the brain. In the present study, we aimed to investigate the differences in cortical thickness according to specific types of childhood abuse between patients with major depressive disorder (MDD) and healthy controls (HCs).
Methods
A total of 61 patients with MDD and 98 HCs were included in this study. All participants underwent T1-weighted magnetic resonance imaging, and the occurrence of childhood abuse was assessed using the Childhood Trauma Questionnaire. We investigated the association between whole-brain cortical thickness and exposure to any type of childhood abuse and specific type of childhood abuse in the total sample using the FreeSurfer software.
Results
No significant difference was reported in the cortical thickness between the MDD and HC groups nor between the “any abuse” and “no abuse” groups. Compared to no exposure to childhood sexual abuse (CSA), exposure to CSA was significantly associated with cortical thinning in the left rostral middle frontal gyrus (p=0.00020), left (p=0.00240), right fusiform gyri (p=0.00599), and right supramarginal gyrus (p=0.00679).
Conclusion
Exposure to CSA may lead to cortical thinning of the dorsolateral prefrontal cortex, which is deeply involved in emotion regulation, to a greater extent than other types of childhood abuse.
INTRODUCTION
Childhood abuse is defined as “a behavior toward a child that is outside the norms of conduct and entails substantial risk of causing physical or emotional harm.” [1] According to the World Health Organization, one in five women and one in 13 men reported having been sexually abused as a child at the ages of 0–17 years, and nearly 300 million children, aged 2–4 years, are regularly physically or emotionally abused by their caregivers [2]. This statistic is particularly concerning as childhood abuse significantly increases the risk of developing depression and is associated with treatment-resistant depression with a chronic course [3]. A previous study demonstrated that the severity of childhood abuse, assessed using the Childhood Trauma Questionnaire (CTQ), was positively associated with the diagnosis of depression and severity of depression symptoms in the victims [4]. Therefore, there is an urgent need to investigate how childhood abuse is associated with depression to implement targeted and effective early intervention programs to reduce the debilitating effects of childhood abuse.
The human brain is a structurally and functionally complex system that undergoes modifications in response to various external stimuli [5]. Adverse experiences such as childhood abuse could impact the structural and functional development of the brain over time, making the victims highly vulnerable to depression [6]. Neuroimaging studies indicates structural alteration of brain regions involved in emotion regulation, such as lateral and medial parts of prefrontal cortices (PFC), anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), insula, and several parietal and temporal regions, in individuals with a history of childhood abuse [6-9]. Cortical thinning in these regions may reflect a decline in the number of neuronal cells, cell alterations, and impaired neuronal interconnectivity [5]. Although the underlying neurobiological changes are not fully understood, it is reasonable to assume that childhood abuse serves as a potent threat cue that activates the physiological stress response system, i.e., the hypothalamic-pituitary-adrenal (HPA) axis, and upregulates the epigenetic process of related genes, leading to elevated baseline cortisol levels [10]. Continuous and repeated childhood abuse leads to prolonged elevation of cortisol levels, which can have neurotoxic effects on the brain, especially on vulnerable cortical regions, such as the PFC, which has a high density of glucocorticoid receptors [10,11]. This fact is supported by prior studies that demonstrated that childhood abuse is associated with reduced volume in the ACC and PFC, regions consistently found to be affected in patients with major depressive disorder (MDD) [12].
Researchers propose that different types of childhood abuse may induce different functional changes in the brain, suggesting a distinct neurobiology of specific types of childhood abuse [13]. Childhood abuse can be categorized into abusive type and neglectful type, and the abusive type can be further categorized into three subtypes: childhood emotional abuse (CEA), childhood physical abuse (CPA), and childhood sexual abuse (CSA) [14]. In general, childhood abuse is associated with an increased responsiveness to affect-based stimuli, consistent with hypervigilance to threat, and neglectful abuse, with an increased responsiveness to general environmental stimuli, consistent with emotional numbing [13]. Specifically, CPA is associated with increased responsiveness to threat in the dorsomedial PFC and lateral PFC, which are associated with attentional processing, while CSA is associated with increased responsiveness to salient visual stimuli in regions of the brain involved in the representation of emotional valence [13]. Few demonstrated CSA-related brain structural changes involve reduction in the volumes of the occipital cortex, hippocampus, and corpus callosum and attenuated cortical development [12,15]. Rinne-Albers et al. [16] showed a significantly smaller volume of the dorsal ACC in a CSA-related post-traumatic stress disorder group than in healthy controls (HCs), suggesting that the involvement of the dorsal ACC in CSA-related neurobiological changes can explain the aberrant emotional processing in traumatized adults that have been exposed to CSA. A recent meta-analysis investigated the impact of the severity and type of childhood maltreatment (categories of “no childhood maltreatment,” “only neglect,” “neglect and abuse”) and found that compared to “no childhood maltreatment,” both types of childhood maltreatment were associated with reduced cortical thickness in the superior temporal sulcus and supramarginal gyrus, and “neglect and abuse” was associated with reduced thickness in inferior parietal lobe, middle temporal lobe, and precuneus [9]. Despite emerging studies on this issue, little research has been conducted on the effect of specific types of childhood abuse on cortical thickness in patients with MDD.
Thus, in the present study, we aimed to investigate the differences in cortical thickness according to specific types of childhood abuse in patients with MDD and HCs. We also explored the differences in cortical thickness between patients with MDD and HCs. Our a priori hypotheses were as follows: 1) childhood abuse will be associated with cortical thinning in the PFC, ACC, OFC, or insula, all of which are involved in emotion regulation, regardless of the diagnosis of MDD, according to the results of previous neuroimaging studies [6,8,9]; 2) according to previous studies on CSA-related structural changes in the brain cortices, individuals exposed to CSA will show more prominent findings than individuals exposed to other types of childhood abuse [12,15,16].
METHODS
Participants
This study included 61 patients with MDD and 98 HC participants. Patients were recruited from the outpatient psychiatric clinic of the Korea University Anam Hospital in Seoul, Republic of Korea, between May 2019 and February 2021. The patients included in this study were adults aged 19–64 years. One of the authors (Han KM), a board-certified psychiatrist, confirmed the diagnosis of MDD using a Structured Clinical Interview for the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders [17]. The present study adopted the following exclusion criteria, similar to those in our previous studies [18,19]: 1) comorbidity with any other major psychiatric disorders (including personality and substance use disorders), 2) MDD with psychotic features, 3) acutely suicidal or homicidal patients requiring inpatient treatment, 4) history of major medical conditions, 5) primary neurological illness, and 6) contraindication for magnetic resonance imaging (MRI). The illness duration of patients with MDD was assessed using the life-chart methodology. In the HC group, 98 healthy volunteers aged 19–64 years were recruited from the community using advertisements. A board-certified psychiatrist assessed the HCs and confirmed that none of them had any current or previous psychiatric disorder. The same exclusion criteria used for the MDD group were applied to the HC participants. The severity of depressive symptoms of all participants was assessed using the 17-item Hamilton Depression Rating Scale (HDRS) on the day of the MRI scan [20]. All participants were confirmed to be right-handed according to the Edinburgh handedness test [21]. The study protocol was approved by the Institutional Review Board of Korea University Anam Hospital (IRB No. 2019AN0174). In accordance with the Declaration of Helsinki, all participants provided written informed consent before participation.
Assessment of childhood trauma
The CTQ was administered to assess childhood abuse and neglect. The CTQ has been widely used in empirical research on patients with psychiatric disorders and this instrument consists of 28 questions that assess childhood maltreatment histories of adults with five dimensional subtypes: CEA, CPA, CSA, emotional neglect, and physical neglect [14,22]. The present study focused on the three subtypes of childhood abuse: CEA, CPA, and CSA. The participants were assigned to the “any abuse group,” if they scored above the moderate-severe cutoff scores, as established by Bernstein and Fink [1], in any of the childhood abuse subtypes: ≥13 for CEA, ≥10 for CPA, and ≥8 for CSA. The participants who did not score above the moderate-to-severe cutoff scores in any of the three subscales were assigned to the “no abuse” group.
MRI data acquisition
Structural T1-weighted images were obtained parallel to the anterior commissure-posterior line using a 3T Siemens scanner (Siemens Healthineers, Munich, Germany) at the Korea University Brain Imaging Center (Seoul, South Korea). Whole-brain T1-weighted images were collected for each participant using magnetization-prepared rapid gradientecho sequences with the following parameters: repetition time, 1,900 ms; echo time, 2.6 ms; field of view, 220 mm; matrix size, 256×256; slice thickness, 1 mm; coronal slices without gap, 176; voxels, 0.86×0.86×1 mm2 ; flip angle, 16°; and number of excitations, 1.
Image processing
Cortical reconstruction was performed using FreeSurfer 7.1.1 version (http://surfer.nmr.mgh.harvard.edu; Laboratory for Computational Neuroimaging, Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, USA). Details of these procedures have been published elsewhere [18,23-30]. Fully segmented images and cortical thickness maps were produced by applying the semi-automated segmentation tool in FreeSurfer to MRIs. 23 The automatically segmented gray/white matter boundaries of the reconstructed brain images were inspected visually and inaccurate data were discarded. The data were smoothed using a Gaussian kernel at full width at half maximum of 15 mm. The cortical thickness of each brain region was calculated by implementing the FreeSurfer algorithm by averaging the distances between the estimated pial surface and the gray-white boundary surface, using the surface deformation algorithm.
Statistical analyses
To compare the cortical thickness between the MDD and HC groups, whole-brain vertex-wise general linear model (GLM) was applied using FreeSurfer version 7.1.1, controlled for the effects of age, sex, and years of education. Further, the GLM was used to investigate the differences in cortical thickness according to childhood abuse exposure in the total sample. The differences in cortical thickness between the “any abuse” and “non-abuse” groups were compared, with age, sex, years of education, and HDRS score as covariates. Subsequently, differences in cortical thickness were analyzed for each subtype of childhood abuse using the same method. To correct the multiple comparison problem, a precomputed Z-Monte Carlo simulation implemented in FreeSurfer was applied. The cluster-forming threshold was 0.001, as recommended by Greve and Fischl [31], and the cluster-wise p-value was 0.05. The sociodemographic and clinical characteristics of the MDD and HC groups were assessed using independent t-tests and chi-square tests; all analyses were conducted using IBM SPSS Statistics for Mac, Version 26.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Comparison of sociodemographic and clinical characteristics of the MDD and HC groups
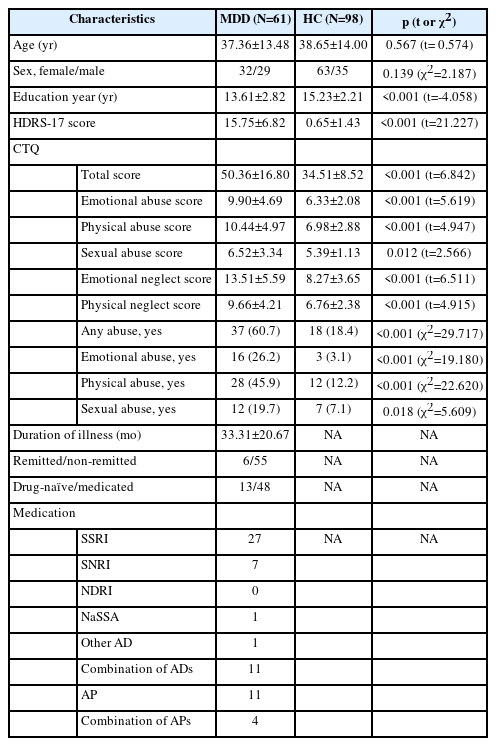
The information on age, sex, years of education, HDRS score, duration of illness, use of psychotropic medication, CTQ scores, and distribution of abuse exposure is presented in Table 1. No significant differences were reported in the age and sex between the two groups, but significantly more years of education were reported in the HC group than in the MDD group (Table 1). Patients with MDD showed significantly higher HDRS and CTQ total and subscale scores (Table 1) along with a significantly higher proportion of exposures to any type of childhood abuse than the HCs (Table 1). Among the 61 patients with MDD, 13 were drug-naïve and 48 were taking psychotropic medications at the time of study enrollment.
Cortical thickness difference according to the diagnosis
In the whole-brain analysis using the GLM, we observed no significant difference in the cortical thickness between the MDD and HC groups, when the effects of age, sex, and years of education were controlled for.
Cortical thickness difference according to childhood abuse exposure
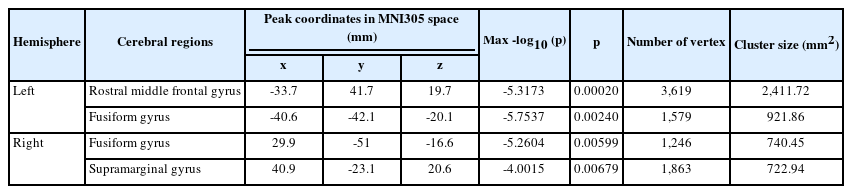
The whole-brain analysis according to any type of childhood abuse, adjusted for age, sex, education year, and HDRS score, showed no significant difference in the cortical thickness between the “any abuse” and “no abuse” groups. In the whole-brain analysis, we observed a significant difference in the cortical thickness according to the CSA exposure. Compared to those with no exposure to CSA, those exposed to CSA showed significant cortical thinning in the left rostral middle frontal gyrus (p=0.00020), left (p=0.00240) and right fusiform gyri (p=0.00599), and right supramarginal gyrus (p=0.00679), when the analysis was controlled for age, sex, education year, and HDRS score (Table 2 and Figure 1). In the whole-brain analyses, no significant difference in the cortical thickness was found according to the CEA or CPA subtypes.
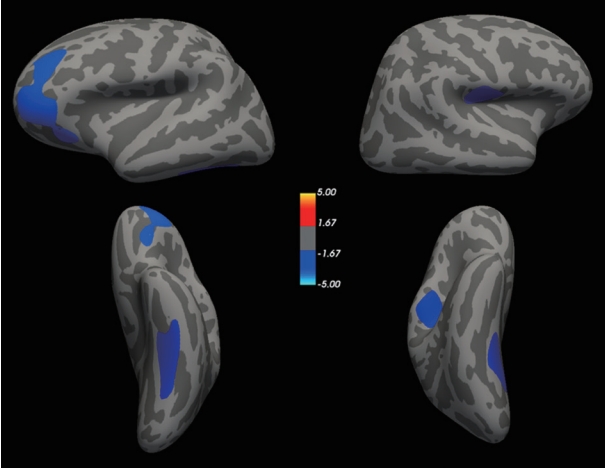
Cortical thinning in participants with history of childhood sexual abuse (CSA) compared to those without history of CSA. The color spectrum bar represents maximum z scores in each cluster for the comparison of cortical thickness between those with and without any history of CSA as per Monte-Carlo permutation cluster analysis.
Correlation between CTQ score and cortical thickness
The correlation between the CTQ-CSA scores and cortical thickness was examined in the total sample by exploratory analysis. In the whole-brain correlation analysis, no statistically significant correlation was observed between the CTQ-CSA scores and cortical thickness. Furthermore, no statistically significant correlation was observed between the CTQ-CSA scores and cortical thickness of the regions of interest, which showed a significant cortical thinning in individuals exposed to CSA.
DISCUSSION
In the present study, we observed that CSA was associated with a significant cortical thinning in the left rostral middle frontal gyrus, which corresponds to the dorsolateral PFC (DLPFC), right supramarginal gyrus, and bilateral fusiform gyrus in the total sample of patients with MDD and HCs. Previous studies have shown that childhood abuse and the diagnosis of MDD are both associated with a reduced cortical thickness in the brain regions involved in emotion regulation, such as the PFC, ACC, OFC, and insula, presenting a neurobiological model that can explain the close relationship between childhood abuse and the development of MDD [6,8,9]. However, emerging studies have shown inconsistent findings, possibly due to differences in the age of onset, sex, severity, and type of childhood abuse [9,13]. Particularly, specific types of childhood abuse have been found to cause structural and functional alterations in the brain in distinct ways [13]. However, little research has been conducted on this topic. To our knowledge, this is the first study to investigate the association of specific types of child abuse with cortical thickness in patients with MDD and HCs.
This study generated three main findings. First, CSA exposure was associated with significant cortical thinning in the total sample. The affected regions included the left rostral middle frontal gyrus, bilateral fusiform gyri, and the right supramarginal gyrus. The rostral middle frontal gyrus corresponds to the DLPFC, which has been consistently associated with childhood abuse in prior research [9]. The DLPFC is responsible for general executive functioning and plays a crucial role in the cognitive regulation of emotion, which involves reinterpreting the meaning of a given emotional stimulus and thereby controlling the intensity of emotional activity [6,32]. Thus, cortical thinning in this region may be related to a disrupted cognitive regulation of emotion. Previous studies have demonstrated that the DLPFC is a key component of the neural network involved in top-down emotion regulation, and disruption of the neural network is a leading cause of cognitive dyscontrol over emotion in the presence of depression [33,34]. Moreover, the DLPFC is functionally connected with the ACC and parietal cortex, forming a so-called cognitive control network (CCN), and the diminished connectivity within the CCN can lead to ineffective cognitive control over emotional regulation [34]. Furthermore, neuroimaging studies have shown that abnormal functioning of the DLPFC leads to disrupted cortical regulation of the limbic system in patients with depression. In a previous study, gray matter volume reduction in the DLPFC was observed in individuals exposed to childhood abuse [35,36], thus linking childhood abuse to the reduction in the cortical thickness of the DLPFC and its disrupted functioning in the process of cortical emotional regulation. Therefore, CSA-associated cortical thinning in the DLPFC can lead to disruption of emotional regulation. Our results show that exposure to CSA, regardless of MDD, could be associated with disrupted emotion processing, posing a greater risk of psychiatric illnesses, including MDD, among those exposed to CSA.
Second, MDD diagnosis was not associated with cortical thinning in our study, in contrast with the results of a previous meta-analysis, which may be due to the smaller sample size of the current study (n=159) than that of the previous meta-analysis (n=10,105) [37]. However, the most recent meta-analysis also showed no effect of MDD diagnosis on the cortical thickness of the participants [9], suggesting that the null finding of the present study indicates that previously reported effects of MDD on cortical thinning may be the result of the interaction of variables such as the onset or duration of childhood maltreatment.
Finally, there was no significant difference in cortical thinning between the “any abuse” and “no abuse” groups in the total sample. This finding is inconsistent with the results of a prior study [9], which showed the severity of childhood maltreatment was associated with the mean cortical surface area, regardless of the region, across all MDD patients and HC participants; the severity of childhood maltreatment was associated with significant thinning in the cortex of several regions in the parietal and temporal lobes. A previous study by Kelly et al. [38] also demonstrated that the severity of childhood abuse was significantly correlated with a reduction of the cortical surface in the left middle temporal area and lingual gyrus. We may have been unable to replicate the effect of childhood abuse on cortical thickness because of the small sample size in the present study. On the other hand, in the study by Tozzi et al. [9], has showed that a history of severe childhood maltreatment, regardless of the MDD diagnosis, was associated with increased thickness in the rostral ACC, which are regions involved in emotional and inhibitory processes. Whether this increase in cortical thickness reflects the adaptive change indicating the resilience of survivors or reactive change as an adverse consequence is unknown, showing the complex impact of childhood maltreatment on the cortical structure of the brain [9,39].
Despite the significant strength of this study as one of the first studies to investigate the effect of specific types of childhood abuse on cortical thickness, there were several limitations. First, we relied on a relatively small sample size (n=159), which may have contributed to the null findings in the present study. Second, 78.7% of the patients with MDD in the present study were taking psychotropic medications, including antidepressants and antipsychotics, which may have affected the results. Third, specific aspects about the experience of childhood abuse, such as onset time (i.e., when they were first exposed to childhood abuse) or duration of childhood abuse, may moderate the effect of childhood abuse on structural brain alterations. Finally, for the assessment of childhood abuse, the present study used the CTQ, which is a retrospective measure and is not free from inaccuracies due to recall bias [40]. Therefore, we cannot exclude the possibility that this may have caused biased results in the present study.
In summary, we identified a significant association between CSA and reduction in cortical thickness in the left rostral middle frontal gyrus, bilateral fusiform gyri, and right supramarginal gyrus, regions involved in the process of emotion regulation, suggesting that exposure to CSA may disrupt the normal process of emotion regulation, to a greater extent than other types of abuse, which may increase the risk of psychiatric disorders such as MDD. Future studies should include a larger sample and more detailed information about childhood abuse to provide deeper insights into the association between specific types of childhood abuse and cortical thickness.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are not publicly available due to containing study participants’ private information but are available from the corresponding author on reasonable request.
Conflicts of Interest
Kyu-Man Han, a contributing editor of the Psychiatry Investigation, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions
Conceptualization: Jisoon Chang, Kyu-Man Han. Data curation: Youbin Kang, Wooyoung Kang, Aram Kim. Formal analysis: Chanju Lee. Funding acquisition: Kyu-Man Han. Investigation: Jinyi Kim, Chanju Lee, Woo-Suk Tae, Byung-Joo Ham, Jisoon Chang, Kyu-Man Han. Methodology: Chanju Lee, Kyu-Man Han. Project administration: Kyu-Man Han. Resources: Kyu-Man Han. Software: Chanju Lee, Kyu-Man Han. Supervision: Jisoon Chang, Kyu-Man Han. Validation: Kyu-Man Han. Visualization: Chanju Lee. Writing—original draft: Jinyi Kim. Writing—review & editing: Jisoon Chang, Kyu-Man Han.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2022R1A2C4001313).