Remimazolam Supplemented to General Anesthesia Alleviates Stress and Cognitive Impairment in Elder Patients After Hip Surgery
Article information
Abstract
Objective
Stress and cognitive impairment are common postoperative complications in elder patients who have undergone hip surgery. The objective of the work is to evaluate the effects of remimazolam supplemented to combined general anesthesia in improving stress and cognitive performance.
Methods
A total of 120 patients were included to receive a low dose of remimazolam (0.1 mg/kg/h) intravenously combined with general anesthesia or general anesthesia alone during hip surgery. Assessments were used for evaluating cognitive and psychological performance respectively before surgery (T0), 24 h (T5), and 72 h (T6) after surgery. Physiological parameters including mean artery pressure, heart rate, and blood oxygen levels (SpO2) were measured at T0, 30 min after anesthesia (T1), and completion of surgery (T2). Stress indexes including serum cortisol and norepinephrine levels were measured at T0, T5, and T6. The visual analog scale pain scores were also acquired at 6 h after surgery, 12 h after surgery, and T6. Serum interleukin-6 and tumor necrosis factor-α levels were acquired at T0, T2, and T6.
Results
Heart rate and SpO2 levels in the combination group were significantly improved compared to the control group. Serum cortisol and norepinephrine levels were the highest at T1 and decline over time until T5 in both groups, the two stress indexes of the combination group were significantly lower at T1 and T2.
Conclusion
Remimazolam supplemented to combined general anesthesia demonstrated significant benefit in reducing stress and cognitive dysfunction in elder patients who underwent hip surgery.
INTRODUCTION
With the elderly population growing worldwide, hip surgery has increasingly become a common procedure to correct joint deformity, relieve joint pain, and improve joint motor function after hip fracture [1]. General anesthesia is one of the commonly adopted procedures in hip surgery [2]. It was reported that postoperative cognitive dysfunction has an incidence rate as high as 38% among elderly surgical patients who have multiple underlying diseases and a low body resistance [3]. As patients remain conscious during operation, anxiety may rise, along with altered hemodynamics and heightened inflammatory responses, contributing to stress and psychological disorders [4]. Therefore, there is an unmet need for optimized anesthesia procedures to minimize postoperative operations.
Despite the recent development of new strategies, e.g., the use of supplementations [5,6], to reduce these complications, none of these strategies have been adopted clinically due to a lack of clinical data. Remimazolam is an ultrashort-acting intravenous sedative agent that arises out of the so-called soft drug development, i.e., the design of active compounds that are rapidly transformed into inactive metabolites in the body.7 Remimazolam undergoes organ-independent metabolism by tissue esterases into an inactive metabolite, and remimazolam is quickly eliminated from the body by first-order pharmacokinetics (with a steady-state half-life of 7–8 min after a 2 h infusion) [8,9]. Due to the lower risk of extended accumulation after lengthy infusion, remimazolam is favored for anesthesia in cardiac surgery and emergency care settings. In a previous study [10], remimazolam was adopted to replace propofol as anesthetic agents to study their anesthetic, stress-reducing, and pain-relieving effects among elderly patients who underwent elective hip replacement, and demonstrated improvement of this combination treatment in terms of postoperative cognitive function compared to propofol anesthesia. In studying colonoscopy diagnosis and treatment, Chen et al. [11] indicated that remimazolam exerted equivalent anesthetic effects but better safety profile compared to propofol. These data implicated that remimazolam may help improve the cognitive function of elderly patients but remimazolam has not been tested as supplementation to general anesthesia. We hypothesize that remimazolam as a supplementation, could reduce stress and improve the cognitive function of elderly patients who underwent hip surgery. Our study strived to test this hypothesis and potentially develop this new anesthesia plan to improve the clinical outcome of these patients.
METHODS
Patient recruitment
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Hefei Second People’s Hospital (#2022-056). Written informed consent to participate in the study were obtained from participants. The following inclusion and exclusion criteria were used.
• Inclusion criteria: patients should be 1) ≥60 years old at the time of admission; 2) undergone elective hip arthroplasty; 3) diagnosed as class I, II, or III according to the American Society of Anesthesiologists (ASA) classification; 4) having no previous history of hip surgery; 5) having no analgesic or sedative medication on 1 day before surgery; 6) no allergy to the anesthetic drugs used in this study; 7) able to cooperate in completing the scale; 8) evaluated preoperatively using Montreal Cognitive Assessment (MoCA) with scores ≥23; 9) evaluated preoperatively using Mini-Mental State Examination (MMSE) with scores ≥23; and 10) having informed consent form signed by the patient and family members.
• Exclusion criteria: patients should not have 1) psychiatric disorders; 2) central nervous system disorders; 3) long-term medications affecting the nervous and psychiatric systems; 4) severe visual, hearing, and speech impairment; 5) allergy to the anesthetic drugs in this study; 6) a history of a severe brain tumor or brain surgery; and 7) severe liver and kidney function and metabolic abnormalities.
The required sample size in the trail was calculated using PS software (https://powerandsamplesize.com; power and sample size collection). The limited sample size was 47 for each group. The patients were divided into a control group (general anesthesia) and an observation group (combined application of remimazolam anesthesia and general anesthesia) by a computer-generated random number table, with 60 cases in each group. The patients were blinded to the treatment and the data analyzers were blinded to the grouping.
Anesthesia procedures
In the control group, patients were first monitored for pulse oximetry (SpO2), blood pressure, and electrocardiogram using a multifunctional monitor (S/5 anesthesia monitor; GE, Chicago, IL, USA), and patients were given rehydration and oxygen. General anesthesia induction in control group was induced by intravenous injection of midazolam (0.04 mg/kg), remifentanil (0.05–1 μg/kg), propofol (1–2 mg/kg), and cis-atracurium (0.1–0.15 mg/kg).
In the combination group (remimazolam anesthesia combined with general anesthesia), remimazolam methylphenidate (Jiangsu Hengrui Pharmaceutical Co, Jiangsu, China) was slowly administered intravenously at the dose of 0.3 mg/kg according to the previous report [12]. After the patient’s conjunctival reflex disappeared, remimazolam of 0.1 mg/kg/h was continuously administered by an intravenous pump. The following time points were used for various analyses: preoperative (T0), 30 min after anesthesia (T1), postoperative (T2), 6 h postoperative (T3), 12 h postoperative (T4), 24 h postoperative (T5), 72 h postoperative (T6).
Study dropouts
Reasons for dropout are as follows: 1) subjects who requested to withdraw from the trial; 2) those who had to stop due to serious adverse effects; 3) those who had a cumulative time of >15 min for blood pressure <90/60 mm Hg or >160/110 mm Hg during the procedure; and 4) those who were unable to complete or refused to complete the entire test, those who were in shock or bradycardic during the procedure, and seven and nine cases were terminated during the study, respectively. Finally, 53 cases in the combination group and 51 cases in the control group were included for analysis.
MoCA and MMSE analysis
Cognitive and mental analyses were performed using MoCA and MMSE scoring systems, respectively. MoCA analysis includes a total score of 30 points in the cognitive domains of attention and concentration, memory, executive function, visual structure skills, language, abstract thinking, calculation, and orientation, with 26–30 points being normal. MMSE includes immediate memory, temporal orientation, place orientation, delayed memory, language, visual-spatial, attention, and calculation, with a total score of 30 points, with 27–30 points being normal, 21–26 points being mildly retarded, 10–20 being moderately retarded, and 0–9 being severely retarded.
Analysis of pain score
The visual analog scale (VAS) scoring system was used to assess the level of pain. The patient was asked to face the scale and mark the corresponding position on the ruler that represents the degree of pain of the patient.
Statistical analysis
Values were expressed as number (%) or mean± standard deviation. p-values were derived from Mann–Whitney test. Chi-square test or Fisher’s exact test was used for assessing the distribution of observations or phenomena between different groups.
RESULTS
Baseline characteristics and physiological data
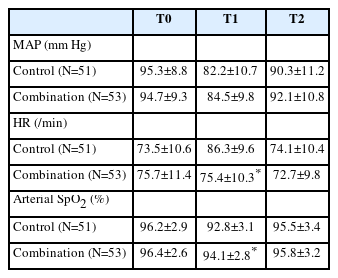
Table 1 summarizes the baseline data of the patients. No significant differences were found between the two groups in terms of ASA classification, age, and sex. There was also no significant difference in the effect of the two types of anesthesia on operation time and intraoperative bleeding.
The mean arterial pressure, heart rate, and oxygen saturation of patients in the two groups were measured at three time points: T0, T1, and T2 (Table 2). At T1, the combination group showed a slower heart rate and higher oxygen saturation than the control group.
Stress evaluations
We compared of stress indexes, including serum cortisol and norepinephrine levels, between the two groups at four time points: T0 (Control: 68.43±10.19 ng/mL, Combination: 71.38±12.41 ng/mL for cortisol; Control: 175.46±18.24 pg/mL, Combination: 168.34±19.11 pg/mL for norepinephrine), T1 (Control: 174.32±31.09 ng/mL, Combination: 148.25±28.13 ng/mL for cortisol; Control: 294.23±38.32 pg/mL, Combination: 260.36±33.43 pg/mL for norepinephrine), T2 (Control: 153.48±29.83 ng/mL, Combination: 128.92±26.06 ng/mL for cortisol; Control: 235.48±34.29 pg/mL, Combination: 211.24±31.47 pg/mL for norepinephrine), and T5 (Control: 93.14±20.13 ng/mL, Combination: 82.93±18.25 ng/mL for cortisol; Control: 191.26±29.55 pg/mL, Combination: 178.29±26.57 pg/mL for norepinephrine). While both groups showed an initial increase in stress indexes at T1, which gradually decreased at T2 and T5, the combination group showed significantly lower stress levels compared to the control group at T1 and T2 (p<0.001) (Figure 1). At T5, the two groups demonstrated no significant differences in stress indexes.
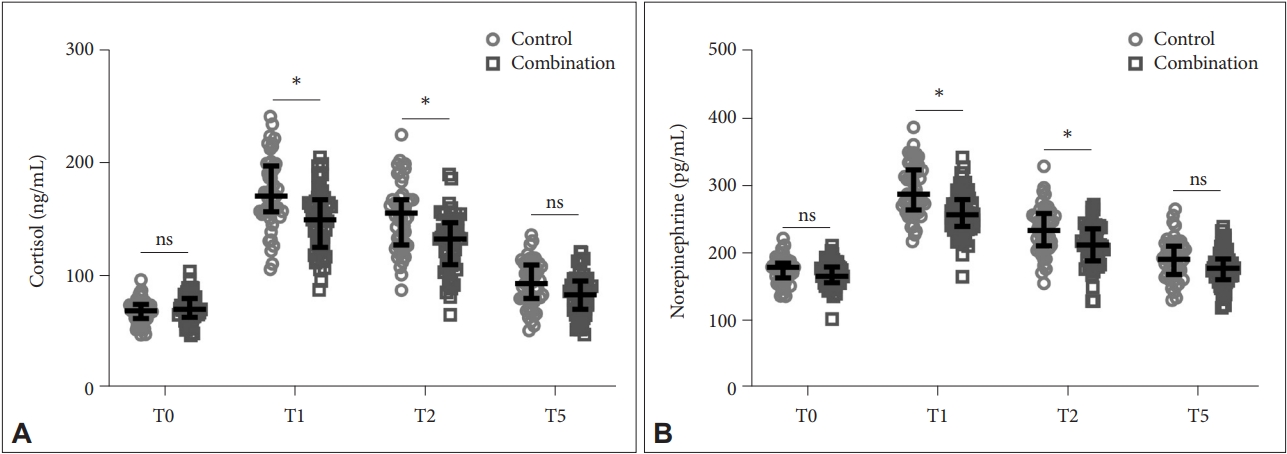
Comparison of stress indexes including serum cortisol (A) and norepinephrine (B) between the two groups at fourtime point. *p<0.001. Data were shown as median with quarterback range. Two-way ANOVA followed by Tukey’s multiple comparisons tests. T0, preoperative; T1, 30 min after anesthesia; T2, postoperative; T5, 24 h postoperative; ns, no significance.
Evaluation of pain levels
The pain levels of patients were conducted at T3 (Control: 4.26±1.16, Combination: 3.41±1.23), T4 (Control: 3.57±0.98, Combination: 2.89±0.99), and T6 (Control: 2.24±0.84, Combination: 1.92±0.97) using the VAS scoring system (Figure 2). We found that there was no significant difference in pain between the two groups at T6, but at T3 and T4, the VAS scores of the combination group were significantly lower than that in the observation group.
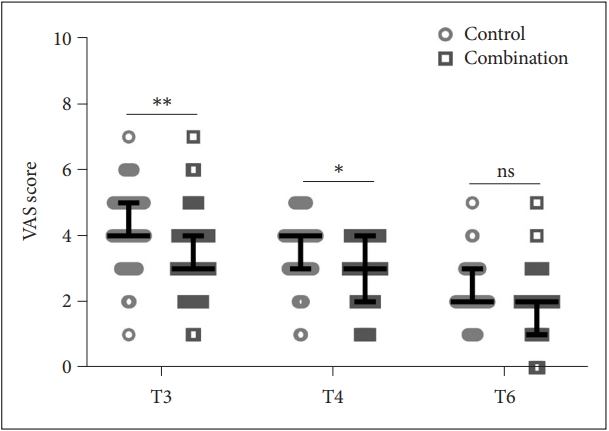
Comparison of VAS scores between the two groups at T3, T4, and T6. *p<0.05; **p<0.001. Data were shown as median with quarterback range. Two-way ANOVA followed by Tukey’s multiple comparisons tests. VAS, visual analog scale; T3, 6 h postoperative; T4, 12 h postoperative; T6, 72 h postoperative; ns, no significance.
Cognitive and mental assessments
Figure 3 shows the comparison of cognitive (Figure 3A) and mental (Figure 3B) impairment between the two groups using MoCA and MMSE analyses, respectively. At T5 (Control: 19.65±3.79, Combination: 22.34±4.24 for MoCA; Control: 19.71±3.23, Combination: 24.21±3.57 for MMSE) and T6 (Control: 22.35±3.52, Combination: 24.72±3.25 for MoCA; Control: 23.39±3.28, Combination: 25.31±2.62 for MMSE), both groups showed a decrease in MoCA and MMSE scores compared to T0 (Control: 26.96±1.83, Combination: 26.69±1.78 for MoCA; Control: 27.16±2.02, Combination: 27.47±1.91 for MMSE). But the combination group had a less cognitive and mental impairment, as suggested by relatively higher scores, compared to the control group at T5 (p<0.001) and T6 (p<0.01).
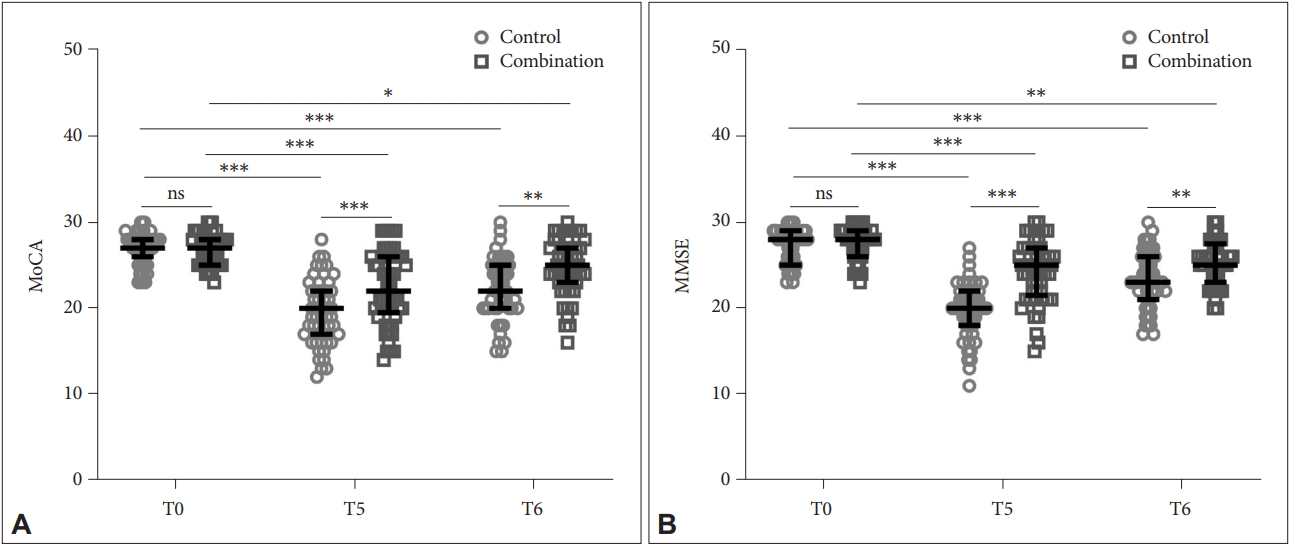
Comparison of Montreal Cognitive Assessment (MoCA, A) and Mini-Mental State Examination (MMSE, B) between the two groups at T0, T5, and T6. *p<0.05; **p<0.01; ***p<0.001. Data were shown as median with quarterback range. Two-way ANOVA followed by Tukey’s multiple comparisons tests. T0, preoperative; T5, 24 h postoperative; T6, 72 h postoperative, ns, no significance.
Analysis of inflammation
Figure 4 shows the comparison of levels of inflammatory factors, including interleukin-6 (IL-6) (Figure 4A) and tumor necrosis factor-α (TNF-α) (Figure 4B) in serum between the two groups. The combination group showed lower levels of IL-6 and TNF-α than the control group at T2 (Control: 195.71±26.34 pg/mL, Combination: 163.66±27.95 pg/mL for IL-6; Control: 118.61±16.26 pg/mL, Combination: 90.33±14.19 pg/mL for TNF-α) and T6 (Control: 145.69±28.41 pg/mL, Combination: 117.58±25.93 pg/mL for IL-6; Control: 72.17±15.79 pg/mL, Combination: 53.27±13.16 pg/mL for TNF-α), suggesting attenuated inflammatory responses.
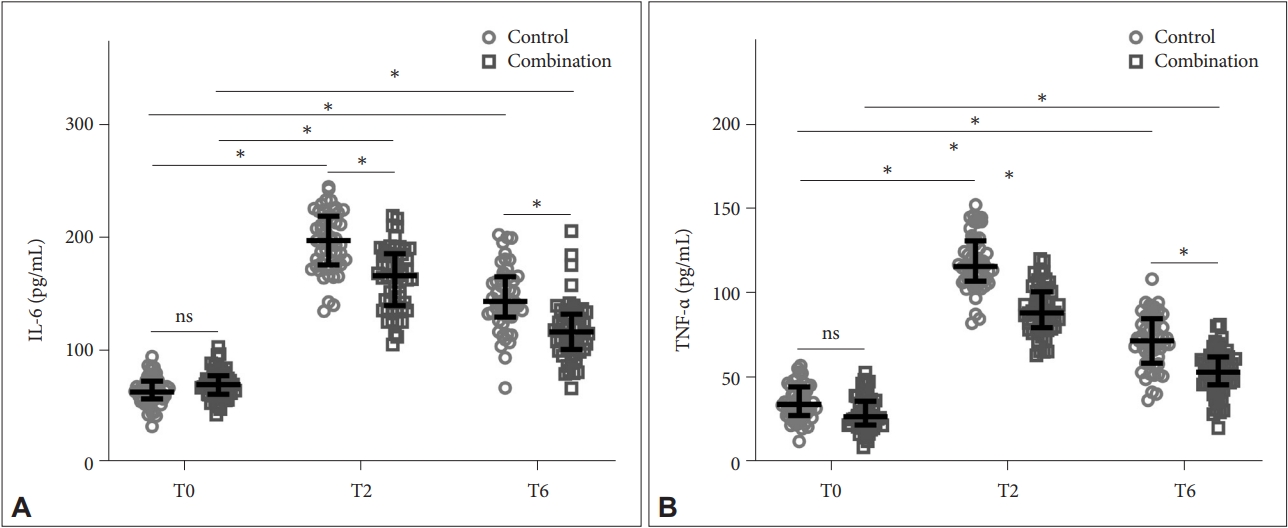
Comparison of serum IL-6 (A) and TNF-α (B) between the two groups at T0, T2, and T6. *p<0.001. Data were shown as median with quarterback range. Two-way ANOVA followed by Tukey’s multiple comparisons tests. IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; T0, preoperative; T2, postoperative; T6, 72 h postoperative; ns, no significance.
DISCUSSION
The rising number of hip surgeries, attributed to a higher proportion of the elderly population and lifestyle change, increasingly necessitates procedural improvement of hip surgery in terms of both efficiency and safety. Unfortunately, postoperative cognitive and mental risks of hip surgery are often ignored and remain to be addressed. The underlying diseases of some elderly patients, such as hypertension and diabetes mellitus, render them more prone to stress reactions due to their poor tolerance of anesthesia and circulatory reserve function [13]. Additionally, the long-term vascular spasm of hypertensive patients may result in aberrant vasculatures, increasing vascular stiffness and decreasing vascular elasticity. Anesthesia may exacerbate the poor vascular elasticity and further lead to poor postoperative outcomes [14]. Therefore, it is particularly critical to developing an ideal anesthesia plan. Remimazolam could act on the inactive metabolites by nonspecific tissue esterases. It does not require dose adjustment in the patients with renal or hepatic impairments, as remimazolam has short context-sensitive half-time, small steady state volume of distribution and high clearance [15]. Additionally, remimazolam has several advantages over propofol, particularly in terms of safety, including no pain on injection, less respiratory depression and hemodynamic instability, and a known reversible agent, flumazenil. Thus, in our study, we aimed to tackle this challenge by focusing on clarifying the benefit of a new anesthesia procedure, which involves the combination of remimazolam and general anesthesia, in hip surgery.
In our study, patients that were epidurally anesthetized along with remimazolam administration showed improved heart rate and oxygen levels, suggesting a relatively better physiological status compared to those without remimazolam administration. Concomitantly, the combination anesthesia also led to lowered serum cortisol and norepinephrine levels, indicating reduced stress levels. All these improvements translated to decreased pain, as measured by VAS scores, and improved cognitive/mental wellness, measured by MoCA/MMSE scores. Our results echoed the previous reports showing that anesthesia with remimazolam also led to better pain and stress scores, along with improved physiological parameters both in hip surgery [10] and colonoscopy [11], but our study is the first to exploit remimazolam as a supplement to enhance postoperative outcomes in general anesthesia.
We demonstrated that remimazolam significantly enhanced pain-relieving and inflammation attenuation effects, which could at least partially shed light on the mechanism of action of this procedure. The inadequate analgesic effect has been considered one of the contributing factors to postoperative stress and cognitive impairment because of surgery [16]. The analgesic and sedation effects of remimazolam, as previously reported [17,18], presumably play an important role in pain-relieving, therefore decreasing stress. Surgery is known to upregulate pro-inflammatory cytokines, which is accountable for a variety of negative physical, behavioral, and psychological changes [19]. Additionally, upregulated pro-inflammatory cytokine expression may lead to hyperalgesia and further cause sensitization of the central and peripheral nervous system, affecting cognitive capabilities [20]. In previous studies, remimazolam has been shown to decrease the inflammatory response in LPS-induced endotoxicity [21], reduce neuropathic pain by regulating autophagy and bradykinin receptor B1. The ameliorating effects of remimazolam on hemodynamic stability are also well recognized [22]. All these effects of remimazolam could orchestrate to improve postoperative outcomes in this new anesthesia plan.
In conclusion, our clinical study demonstrated that remimazolam supplemented general anesthesia can help reduce stress, pain and improve the cognitive function of elderly patients who underwent hip surgery. Inflammatory responses are attenuated by remimazolam supplementation. It is worth promoting the application this new combined anesthesia procedure in clinical practice.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Jun Zhang. Data curation: all authors. Project administration: Jun Zhang. Supervision: Jun Zhang. Writing—original draft: all authors. Writing—review & editing: all authors.
Funding Statement
None