Identification of Plasma Biomarkers in Drug-Naïve Schizophrenia Using Targeted Metabolomics
Article information
Abstract
Objective
Schizophrenia (SCZ) is a severe psychiatric disorder with unknown etiology and lacking specific biomarkers. Herein, we aimed to explore plasma biomarkers relevant to SCZ using targeted metabolomics.
Methods
Sixty drug-naïve SCZ patients and 36 healthy controls were recruited. Psychotic symptoms were assessed using the Positive and Negative Syndrome Scale. We analyzed the levels of 271 metabolites in plasma samples from all subjects using targeted metabolomics, and identified metabolites that differed significantly between the two groups. Then we evaluated the diagnostic power of the metabolites based on receiver operating characteristic curves, and explored metabolites associated with the psychotic symptoms in SCZ patients.
Results
Twenty-six metabolites showed significant differences between SCZ patients and healthy controls. Among them, 12 metabolites were phosphatidylcholines and cortisol, ceramide (d18:1/22:0), acetylcarnitine, and γ-aminobutyric acid, which could significantly distinguish SCZ from healthy controls with the area under the curve (AUC) above 0.7. Further, a panel consisting of the above 4 metabolites had an excellent performance with an AUC of 0.867. In SCZ patients, phosphatidylcholines were positively related with positive symptoms, and cholic acid was positively associated with negative symptoms.
Conclusion
Our study provides insights into the metabolite alterations associated with SCZ and potential biomarkers for its diagnosis and symptom severity assessment.
INTRODUCTION
Schizophrenia (SCZ) is a complex and debilitating psychiatric disorder with a worldwide prevalence of approximately 1% [1]. The disease is characterized by various symptoms, including positive symptoms, negative symptoms, depression/anxiety, agitation, and cognitive impairments [2]. Currently, the diagnosis of SCZ is subjective and relies on clinical symptoms presented by the patient [3]. Therefore, identifying plasma biomarkers associated with SCZ is essential to improve diagnostic accuracy and better understand the pathophysiology of the disease. Despite numerous studies have been conducted from different perspectives and techniques, plasma biomarkers with high correlation with SCZ have not been identified [4].
Metabolomics is a technology that allows the simultaneous detection of all metabolites in an organism, including targeted and non-targeted metabolites [5]. In contrast to non-targeted metabolomics, targeted metabolomics allows accurate qualitative and quantitative analysis of metabolites with specific chemical characteristics in biological samples [6]. In recent years, it has emerged as a promising technique for identifying potential biomarkers for various diseases [7,8]. Notably, Lai et al. [9] conducted a review of 35 metabolomics studies, revealing that glutamate, lactate, and citrate could serve as potential biomarkers for epilepsy. Additionally, Hou et al. [10] performed a systematic analysis of 9 metabolomics studies and identified l-glutamine and citrulline as possible biomarkers of diabetic retinopathy. Furthermore, the application of metabolomics techniques in psychiatric disorders has been explored, such as SCZ, bipolar disorder, and depression [11-13].
Previous metabolomics studies have identified several metabolites with potential as SCZ biomarkers, but the results are inconsistent. Wang et al. [14] found phosphatidylcholine (PC) was significantly lower in SCZ patients and showed good performance in the diagnosis of SCZ. Similarly, Weber-Fahr et al. [15] found that PC was significantly reduced in the thalamus of SCZ and was associated with clinical symptoms of SCZ. However, Hamasaki et al. [16] found that PC in SCZ was not significantly altered and was unsuitable as disease biomarkers. The inconsistent results may be due to the lower accuracy of the detection method, the heterogeneity of patients, and the influence of drugs. Previous studies have tended to recruit chronic SCZ patients, and few studies have been conducted on drug-naïve SCZ patients. Therefore, metabolomics studies in drug-naïve SCZ warrants further investigation.
To the best of our knowledge, studies that explore metabolite markers and further analyze their relationship with clinical symptoms in drug-naïve SCZ patients are relatively scarce, and the conclusions are premature. Therefore, additional relevant studies are warranted. In our study, we utilized targeted metabolomic analysis on plasma samples from all participants. We aimed to: 1) identify potential biomarkers associated with SCZ; 2) establish a diagnostic panel with powerful diagnostic performance for SCZ; and 3) explore metabolomic biomarkers related to psychotic symptoms.
METHODS
Subjects
This study recruited 60 subjects from Tianjin Anding Hospital. The inclusion criteria were as follows: 1) diagnosis of SCZ or schizophreniform disorder independently confirmed by two experienced psychiatrists according to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) [17]; 2) age between 18 and 65 years; 3) primary education or above; 4) not previous use of antipsychotic medication; and 5) total score of Positive and Negative Syndrome Scale (PANSS) >60. Exclusion criteria included: 1) other psychiatric disorders; 2) diabetes mellitus, hyperlipidemia, or other severe physical diseases; 3) pregnant or lactating women; 4) history of physical therapy within the past month, such as modified electroconvulsive therapy and transcranial magnetic stimulation; and 5) any factors that prevent participants from providing informed consent or participating in the study. Thirty-six healthy controls (HCs) were recruited from a healthy population in the community. HCs did not meet the diagnosis of any disease according to DSM-5 and had no family history of psychotic disorders.
All participants provided written informed consent, and the study was approved by the Ethical Committee of Tianjin Anding Hospital (No. 2017-03).
Clinical assessment
General information and clinical assessments were collected from all participants at the time of recruitment using a self-designed questionnaire. The psychotic symptoms were assessed using the PANSS by two experienced psychiatrists with uniform training. PANSS is widely used to evaluate the clinical symptoms of SCZ, with higher scores associated with more severe symptoms [18]. In our study, a 5-factor model was used for the analysis, including positive factor, negative factor, cognitive factor, excitatory factor, and depressive factor [19]. The calculated inter-observer correlation coefficient was more than 0.8.
Sample collection
Blood samples were collected from all participants from the antecubital vein after an overnight fast. Then, the blood samples were processed within 4 hours. All blood samples were centrifuged at 1,000g for 15 min at 4°C and the plasma was divided and frozen at -80°C until analysis.
Flow injection analysis/liquid chromatography-mass spectrometry analysis
To determine the plasma metabolites, a targeted metabolomics analysis was performed with the Biocrates MxP Quant 500 (P500) kit (Biocrates, Innsbruck, Austria) according to the protocol. This kit is used for the targeted quantitative detection of 630 metabolites. These metabolites are associated with neuropsychiatric disorders, including acyl-carnitine, amino acid, bile acid, phospholipid, ceramide (Cer), hormone, etc.
First, 10 μL of sample solution was added to each well of a 96-well extraction plate on the filtration site. Then, the samples were derivatized with 5% phenyl isothiocyanate solution. After derivatization, the target analytes were extracted with an organic solvent and then diluted. Finally, the extracts were detected using a 6500 QTRAP instrument (AB Sciex, Singapore) in flow injection analysis mode and liquid chromatography-mass spectrometry mode, respectively.
Quality control
In this experiment, quality control (QC) samples of known concentration were inserted into the sample cohort and subjected to the same preprocessing and mass spectrometry detection process as the samples to be tested. Therefore, the data quality could be assessed based on the precision of each metabolite in the QC samples. A 25% filtering standard was applied.
Statistical analyses
Analyte concentrations and data evaluation were calculated using Biocrates MetIDQ software (Biocrates). The concentrations of all metabolites were normalized based on internal QC samples. Variables with more than 50% missing were removed and those with less than 50% missing were replaced with the arithmetic mean of all valid measurements. Data transformation and scaling were carried out before performing principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) using SIMCA software (version 14.0; Umetrics, Umea, Sweden). The OPLS-DA model was used to obtain the variable importance in the projection (VIP) value. A 200-permutation validation was performed to assess the fitting of the OPLS-DA model [20]. At the same time, t-tests were performed to assess the significance of the variables. Metabolites with VIP >1 and p<0.05 were selected for further identification. Differential metabolites were visualized using heat-maps on the MetaboAnalyst 5.0 platform (http://www.metaboanalyst.ca).
For the clinical variables, group comparisons were used with t-tests for continuous variables and chi-square test for categorical variables. Analysis of covariance (ANCOVA) was used to compare differences in these metabolites between the two groups, with sex, age, and body mass index (BMI) as covariates. The receiver operating characteristic (ROC) curve was used to evaluate the diagnostic efficacy of the metabolites. Prior to correlation analysis, differential metabolites of the same biological type were combined using simple addition. Partial correlation analysis was used to analyze the correlation between the metabolites and psychotic symptoms for SCZ patients, including sex, age, and BMI as covariates. All statistical analyses were performed using IBM SPSS version 24.0 (IBM Corp., Armonk, NY, USA), and p<0.05 was considered significant.
RESULTS
Demographics and clinical characteristics
The demographic and clinical characteristics of all participants were summarized in Table 1. There were no significant differences between SCZ patients and HCs in terms of sex, age, and years of education (all p>0.05). In addition, SCZ patients differed from HCs in marital history and BMI (p<0.05).
Plots of PCA scores showed that metabolite profiles were separated between SCZ patients and HCs (Supplementary Figure 1 in the online-only Data Supplement). One patient was excluded from the subsequent analysis due to significant differences from other patients (Supplementary Figure 2 in the online-only Data Supplement).
Differential metabolites between patients and HCs
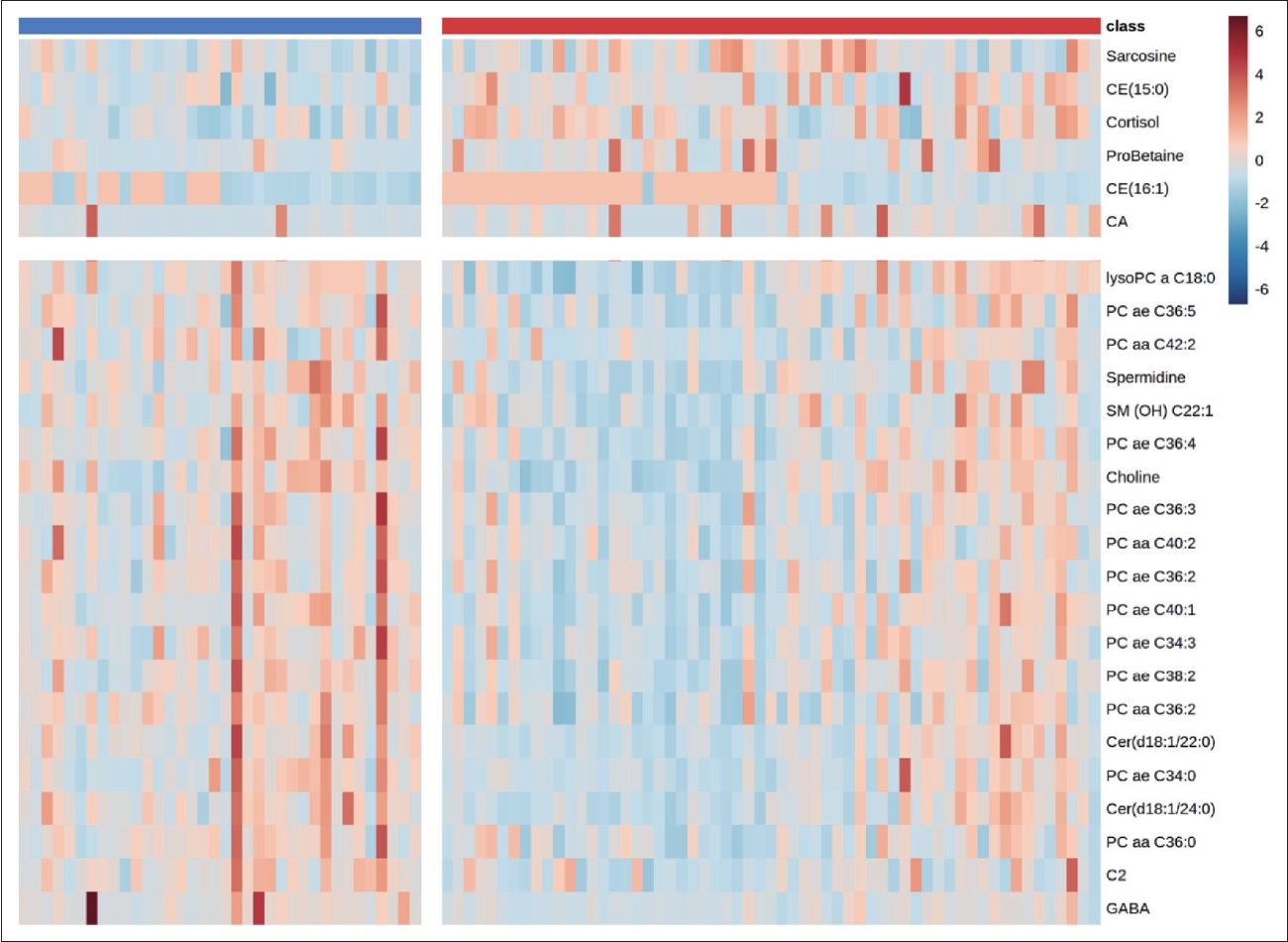
In this study, we measured 271 metabolites. After excluding errors and other factors, 188 metabolites remained. OPLS-DA was used to analyze the metabolic differences between patients and HCs. The score plot showed a clear discrimination between patients and HCs (Figure 1A), with R2Y=0.849 and Q2=0.57 in the model. The supervised models were confirmed with 200 times permutation tests (Figure 1B). After screening based on p<0.05 and VIP >1, 29 metabolites in plasma differed significantly between patients and HCs. After ANCOVA with sex, age, and BMI as covariates, 26 metabolites passed correction and were associated with SCZ. Among them, 12 metabolites were PCs. Figure 2 shows the VIP values of 26 differential metabolites. Compared to HCs, sarcosine, cholesterol ester (CE) (15:0), cortisol, proline betaine (probetaine), CE (16:1), and cholic acid (CA) were upregulated in SCZ patients. The remaining 20 metabolites were downregulated. The heat map shows the distribution of 26 differential metabolites in each sample (Figure 3).
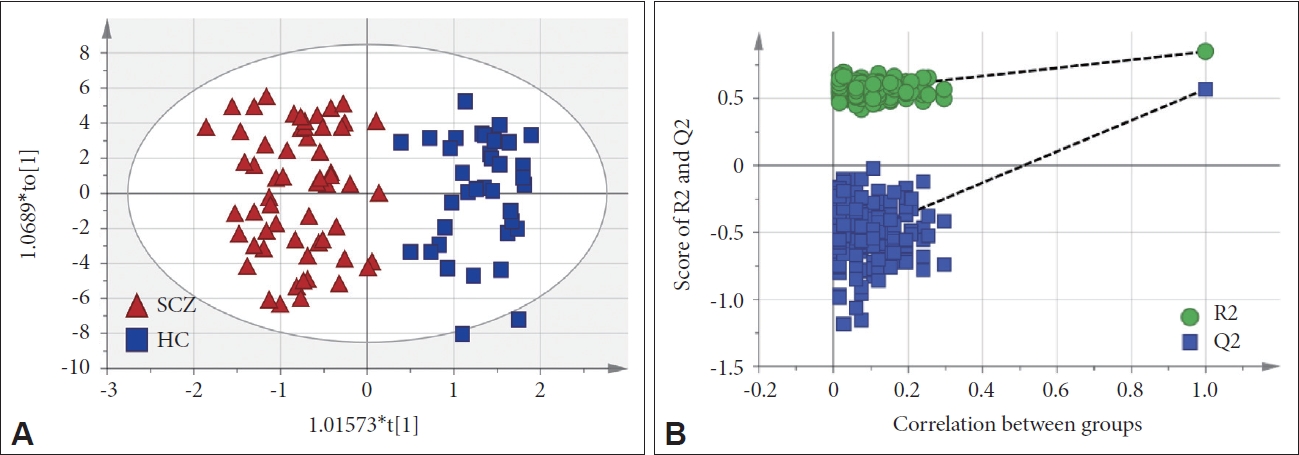
Metabolomic analysis between schizophrenia (SCZ) and healthy control (HC). A: Score plot of orthogonal partial least squares discriminant analysis. B: The plot of permutation validation.
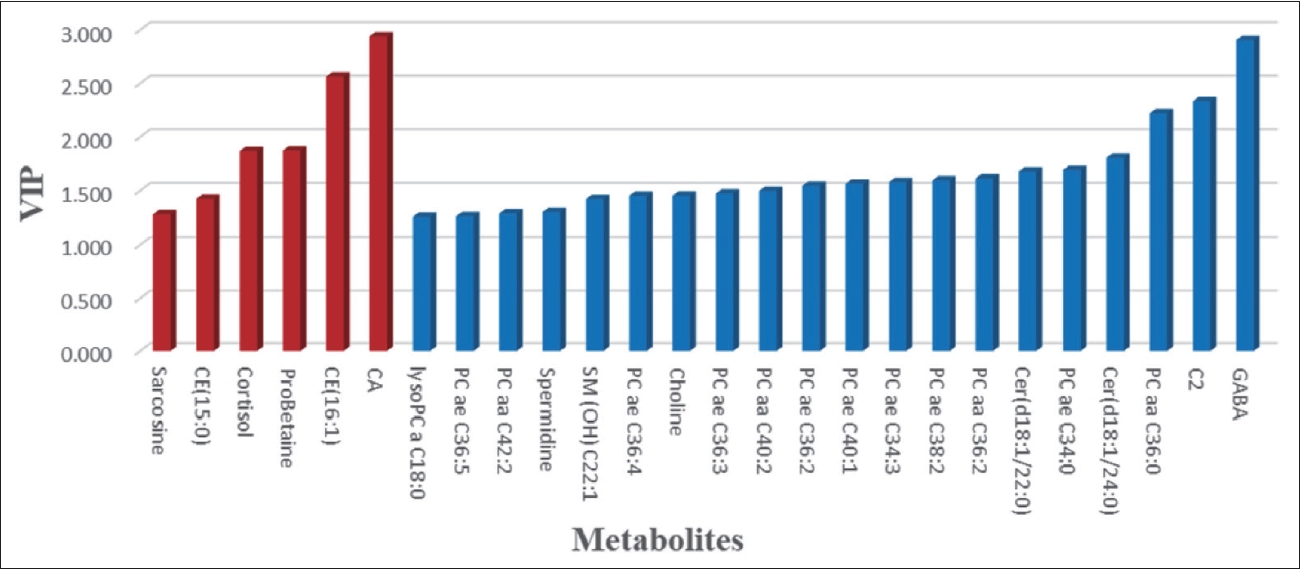
Variable importance in the projection (VIP) values of 26 differential metabolites. Red represents metabolites that are upregulated in the patients compared to the healthy controls. Blue means downward adjustment.
Building diagnostic model using ROC curve
Given that these metabolites were significantly different between the two groups, we tested whether these metabolites have the potential to be diagnostic biomarkers of SCZ. According to the ROC curve, cortisol, Cer (d18:1/22:0), acetylcarnitine (C2), and γ-aminobutyric acid (GABA) showed exemplary performance in diagnosing SCZ, with an area under the curve (AUC) of 0.736, 0.719, 0.736, and 0.773, respectively. The diagnostic panel combining the 4 metabolites had better diagnostic power with an AUC of 0.867 (Figure 4).

Receiver operating characteristic (ROC) curves. A: ROC curves of cortisol, Cer (d18:1/24:0), C2, and GABA, and the corresponding area under the curve (AUC) values are 0.736, 0.719, 0.736, and 0.773, respectively. B: ROC curves for a diagnostic panel consisting of 4 metabolites, and the AUC value is 0.867. Cer, ceramide; C2, acetylcarnitine; GABA, γ-aminobutyric acid.
Associations between plasma metabolites and symptoms
These 26 differential metabolites belonged to 12 biology types. After combining differential metabolites of the same biology type, partial correlation analysis was used to explore the correlation between the metabolites and psychotic symptoms. As shown in Table 2, the levels of plasma CA were positively correlated with the negative factor of PANSS (r=0.292, p=0.029). In addition, positive correlations were observed between plasma PCs levels and positive factor in PANSS (r=0.296, p=0.027).
DISCUSSION
To our knowledge, our study is the first to utilize a targeted metabolomics approach to explore the plasma metabolites associated with SCZ and psychotic symptoms in drug-naïve SCZ. In this study, we identified 26 out of 271 metabolites that were different between the two groups. Among these, 12 metabolites were PCs, suggesting that PCs could be potential biomarkers for SCZ. Additionally, a panel composed of cortisol, Cer (d18:1/22:0), C2, and GABA showed better diagnostic power for SCZ. Furthermore, CA and PCs were associated with clinical symptoms of SCZ.
The present study found a decrease in PC levels in SCZ patients, including 12 subtypes such as C34:0, C36:0, C38:2, and C40:2. This is consistent with previous studies, which have reported aberrant metabolism of PC as a major feature of SCZ [21-23]. Kaddurah-Daouk et al. [24] found a slight downregulation of PC levels in the plasma of SCZ patients. And in an early metabolomics study, Schwarz et al. [25] also found reduced PC in the prefrontal cortex of SCZ patients. However, Wang et al. [14] found that PC levels containing saturated fatty acids or monounsaturated fatty acids side chains were generally increased. The discrepancies between these findings may be attributed to the differences in the disease states of the studied populations, leading to different pathological changes. For example, reduced conversion of PC to acylcarnitine leads to an increase in PC levels [26]. And high levels of the synthetic raw material choline could lead to increased synthesis of PC, which could also lead to increased PC levels [27]. There are other contributing factors to consider as well. For example, it has been observed that PC levels in SCZ patients inherently display subtype-specific differential expression patterns [28]. Consequently, when different studies focus on distinct PC subtypes, it is not uncommon to observe varying results that may seem contradictory. Furthermore, the influence of antipsychotic medication usage and discrepancies in metabolomic assay techniques can also impact the final outcomes of the study. The mechanisms underlying PC metabolic abnormalities in SCZ are not yet fully understood. Hasegawa et al. [29] have indicated that PC is a lipid with a wide range of biological activities that affect inflammatory responses and immune processes. Janssen and Kiliaan [30] found that PC is a reservoir for arachidonic acid, which is the basic precursor of signaling molecules, and disruption of PC levels indirectly affects the signaling process. Further studies are needed to explore the intrinsic mechanisms linking PCs and SCZ.
Moreover, cortisol, Cer (d18:1/22:0), C2, and GABA had good diagnostic power for SCZ. Previous studies have extensively investigated the role of these metabolites in SCZ. Girshkin et al. [31] and Mück-Seler et al. [32] found significantly higher cortisol levels in patients with SCZ, which is consistent with our results. Although cortisol has shown an unusual ability to diagnose depression in HCs, our study provides evidence of its efficacy in diagnosing SCZ, which needs further validation [33]. Smesny et al. [34] found elevated Cer in the stratum corneum of patients with first-episode SCZ. However, our study found a significant decrease in Cer levels in SCZ patients, possibly because we focused more on changes in Cer subtypes rather than total amounts. de Almeida et al. [35] found that lipids such as Cer could be used to differentiate the efficacy response to different antipsychotics, but studies are still needed to verify whether Cer is effective in diagnosing patients with SCZ. Cao et al. [36] and Cui et al. [37] found that medium and long-chain acylcarnitine was significantly lower in SCZ patients, and they also demonstrated that acylcarnitine has an excellent ability to diagnose SCZ, outperforming functional near-infrared spectroscopy as well as uric acid and monoamines, which is consistent with our conclusion. Our study also confirmed a significant reduction in GABA levels in patients with SCZ, consistent with the findings of Chiu et al. [38], who also reported the diagnostic power of GABA. However, the low diagnostic power of these metabolites for SCZ emphasizes the complexity of the pathogenesis, requiring a diagnostic panel consisting of multiple biomarkers to improve diagnosis accuracy.
In our study, a diagnostic panel consisting of cortisol, Cer (d18:1/22:0), C2, and GABA greatly improved the diagnostic ability for SCZ. This strategy of a diagnostic panel has been used in earlier studies. He et al. [39] found that a diagnostic panel consisting of 5 plasma metabolites, including arginine, glutamine, histidine, ornithine, and PC, greatly improved the diagnosis relative to individual metabolites. Xuan et al. [40] performed a metabolomic analysis of plasma from SCZ patients and found that a panel of biomarkers consisting of citrate, palmitic acid, myo-inositol, and allantoin had good diagnostic power, with an AUC of more than 0.9. Using a multidimensional endocrine strategy, a study chose five hormones to create a diagnostic panel, including cortisol, epinephrine, norepinephrine, testosterone, and free thyroxine, to differentiate SCZ from bipolar disorder with satisfactory results [41]. This study introduces, for the first time, a diagnostic panel that holds great potential in identifying SCZ patients at an early stage, enabling early intervention and significantly improving patient prognosis. Previous studies have indicated the promise of plasma metabolites in facilitating the early diagnosis of SCZ [42,43]. Nonetheless, further advancements are required before these findings can be translated into clinical applications. A problem that should not be overlooked is that current metabolite databases are not standardized and different laboratories often use different databases, which makes the diagnostic panel less reproducible across studies and thus affects the generalization of findings. And the results of these studies lack validation of independent samples. Future advances in metabolomics technology and methods with larger independent samples could help overcome this challenge.
Moreover, we identified 2 metabolites, PC and CA, that were associated with clinical symptoms of SCZ. PC levels were positively correlated with positive symptoms, while CA levels were positively correlated with negative symptoms. A magnetic resonance spectroscopy study in the cerebral cortex also found a correlation between PC levels and psychotic symptoms [15]. However, Wang et al. [14] indicated that PC was not significantly associated with PANSS scores. The different results may be due to differences in metabolomics techniques and processing, data analysis, and patient characteristics. Since there is currently no animal model specifically designed to study the relationship between PC and psychotic symptoms in SCZ, we must seek insights from comparable animal models. Notably, in an obese mouse model, Fu et al. [44] revealed that disrupted PC metabolism resulted in the inhibition of sarco/endoplasmic reticulum calcium ATPase activity, a potential mechanism implicated in the development of psychotic symptoms. Furthermore, Li et al. [45] demonstrated in the colitis mouse model that PC was associated with the regulation of specific gut bacteria, including Lactobacillus, Faecalibacterium, Dubosiella, and Turicibacter. For our study, we observed disturbances in intestinal flora among patients with severe psychotic symptoms. While these findings provide valuable clues, further research is necessary to unravel the underlying mechanisms. Similarly, the positive association between CA levels and negative symptoms in our study contrasts with Khosravi’s case report [46], which suggested that ursodeoxycholic acid augmentation therapy was effective in relieving negative symptoms of SCZ. This contrary finding may probably due to the metabolite levels in this case disturbed by the drug. Since there are few similar studies, we want to look at some mechanistic studies on CA to find evidence to support our conclusions. Pollak et al. [47] suggested that excessive CA levels could cause blood-brain barrier dysfunction, which could worsen the symptoms of SCZ. Long et al. [48] suggested that there was an interaction between gut flora microecology and CA levels, and disturbances in CA levels could cause disturbances in gut flora microecology, which could also cause the worsening of SCZ symptoms. Therefore, further studies are needed to investigate the role of CA in SCZ.
Several limitations should be noted in the present study. First, due to the difference in BMI, the HCs could not be matched precisely to the patients. Although we applied ANCOVA to correct this discrepancy, the results still require a better-matched sample to be validated. Additionally, medication use and dietary habits were not controlled for in the study, which could have affected the results. Finally, our findings need to be replicated and validated in future studies with larger and more diverse samples.
In conclusion, our study provides novel insights into the metabolite disturbances in SCZ patients, especially PC. Cortisol, Cer (d18:1/22:0), C2, and GABA showed potential as biomarkers for SCZ, and a panel composed of these metabolites performed well in diagnosing the disease. Furthermore, our findings suggest that PCs and CA are associated with psychotic symptoms, and these metabolites could be potential targets.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.30773/pi.2023.0121.
Score plot of principal component analysis. SCZ, schizophrenia; HC, healthy control.
Hotelling’s T2 range line plot of principal component analysis.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Jie Li. Data curation: Qiao Su. Formal analysis: Fuyou Bi. Funding acquisition: Jie Li. Investigation: Huiming Yan. Methodology: Xiaoxiao Sun. Project administration: Jiayue Wang. Resources: Yuying Qiu. Software: Qiao Su. Supervision: Meijuan Li. Validation: Shen Li. Visualization: Shu Yang. Writing—original draft: Qiao Su, Fuyou Bi, Shu Yang, Shen Li. Writing—review & editing: all authors.
Funding Statement
Funding for this study was provided by grants from Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-033A), Tianjin Key Medical Discipline (Specialty) Construction Project of Tianjin Health Commission (TJWJ2022XK039), Foundation of Tianjin Health Commission for Young Scholars (KJ20067) and Natural Science Foundation of Tianjin Municipal Science and Technology Bureau (22JCZDJC00110).
Acknowledgements
We thank all of the study participants for their cooperation.